Deflazacort: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 4: | Line 4: | ||
| verifiedrevid = 447631837 | | verifiedrevid = 447631837 | ||
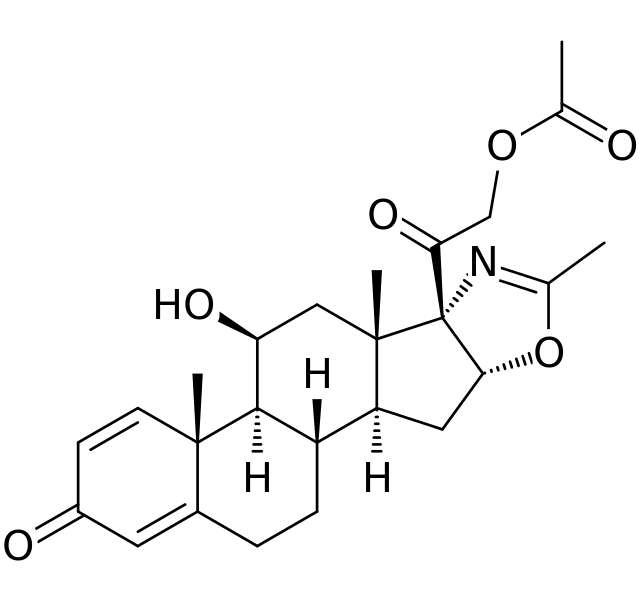
| IUPAC_name = (11β,16β)-21-(acetyloxy)-11-hydroxy-2′-methyl-5′''H''-pregna-1,4-dieno[17,16-''d'']oxazole-3,20-dione | | IUPAC_name = (11β,16β)-21-(acetyloxy)-11-hydroxy-2′-methyl-5′''H''-pregna-1,4-dieno[17,16-''d'']oxazole-3,20-dione | ||
| image = Deflazacort structure. | | image = Deflazacort structure.png | ||
<!--Clinical data--> | <!--Clinical data--> | ||
| Line 53: | Line 53: | ||
| molecular_weight = 441.517 g/mol | | molecular_weight = 441.517 g/mol | ||
}} | }} | ||
__Notoc__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Deflazacort''' is a [[glucocorticoid]] used as an [[anti-inflammatory]] and [[immunosuppressant]]. | '''Deflazacort''' is a [[glucocorticoid]] used as an [[anti-inflammatory]] and [[immunosuppressant]]. | ||
| Line 64: | Line 68: | ||
[[Category:Glucocorticoids]] | [[Category:Glucocorticoids]] | ||
[[Category:Prodrugs]] | [[Category:Prodrugs]] | ||
Revision as of 18:46, 8 April 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 40% |
| Metabolism | By plasma esterases, to active metabolite |
| Elimination half-life | 1.1–1.9 hours (metabolite) |
| Excretion | Renal (70%) and fecal (30%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C25H31NO6 |
| Molar mass | 441.517 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Deflazacort |
|
Articles |
|---|
|
Most recent articles on Deflazacort Most cited articles on Deflazacort |
|
Media |
|
Powerpoint slides on Deflazacort |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Deflazacort at Clinical Trials.gov Clinical Trials on Deflazacort at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Deflazacort
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Deflazacort Discussion groups on Deflazacort Patient Handouts on Deflazacort Directions to Hospitals Treating Deflazacort Risk calculators and risk factors for Deflazacort
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Deflazacort |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Deflazacort is a glucocorticoid used as an anti-inflammatory and immunosuppressant.
Deflazacort is an inactive prodrug which is metabolized rapidly to the active drug 21-desacetyldeflazacort.[1] Its potency is around 70–90% that of prednisone.[2]
It is sold in Bangladesh as Xalcort, marketed by Beacon Pharmaceuticals Limited, in the United Kingdom by Shire under the trade name Calcort;[2] in Brazil as Cortax, Decortil, and Deflanil; in India as MOAID, Defolet(Roussette), DFZ, Decotaz, and DefZot; in Panama as Zamen, Spain as Zamene and in Honduras as Flezacor.[3] It is not available in the United States. In January 2015, the FDA granted fast track status to Marathon Pharmaceuticals to pursue approval of deflazacort as a potential treatment for Duchenne muscular dystrophy. The company expects to make deflazacort available in the United States in 2016. [4]
References
- ↑ Möllmann, H; Hochhaus, G; Rohatagi, S; Barth, J; Derendorf, H (1995). "Pharmacokinetic/pharmacodynamic evaluation of deflazacort in comparison to methylprednisolone and prednisolone". Pharmaceutical research. 12 (7): 1096–100. PMID 7494809.
- ↑ 2.0 2.1 "Calcort". electronic Medicines Compendium. June 11, 2008. Retrieved on October 28, 2008.
- ↑ "Substâncias: DEFLAZACORT" (in português). Centralx. 2008. Retrieved on October 28, 2008.
- ↑ http://www.chicagotribune.com/business/ct-marathon-muscular-dystrophy-drug-0119-biz-20150119-story.html#page=1
- Pages with script errors
- CS1 português-language sources (pt)
- Template:drugs.com link with non-standard subpage
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Glucocorticoids
- Prodrugs