Darunavir ethanolate and cobicistat
{{DrugProjectFormSinglePage |authorTag=Shivani Chaparala M.B.B.S [1] |genericName=Darunavir ethanolate and cobicistat |aOrAn=a |drugClass=combination of darunavir, a human immunodeficiency virus (HIV-1) protease inhibitor and cobicistat, a CYP3A inhibitor |indicationType=treatment |indication=HIV-1 infection in adult patients. |adverseReactions=Darunavir: diarrhea, nausea, rash, headache, abdominal pain, and vomiting.
|blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content)
|fdaLIADAdult=* PREZCOBIX® is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection in treatment-naïve and treatment-experienced adults with no darunavir resistance-associated substitutions. Recommended dosage: One tablet taken once daily with food. Tablets: 800 mg of darunavir and 150 mg of cobicistat.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Darunavir ethanolate and cobicistat in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Darunavir ethanolate and cobicistat in adult patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Darunavir ethanolate and cobicistat in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Darunavir ethanolate and cobicistat in pediatric patients. |warnings===== Hepatotoxicity ====
- During the darunavir clinical development program (N=3063), where darunavir was co-administered with ritonavir 100 mg once or twice daily, drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) was reported in 0.5% of subjects.
- Patients with pre-existing liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities including severe hepatic adverse reactions.
- Post-marketing cases of liver injury, including some fatalities, have also been reported with darunavir co-administered with ritonavir.
- These have generally occurred in patients with advanced HIV-1 disease taking multiple concomitant medications, having co-morbidities including hepatitis B or C co-infection, and/or developing immune reconstitution syndrome.
- A causal relationship with darunavir co-administered with ritonavir has not been established.
- Appropriate laboratory testing should be conducted prior to initiating therapy with PREZCOBIX and patients should be monitored during treatment.
- Increased AST/ALT monitoring should be considered in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases, especially during the first several months of PREZCOBIX treatment.
- Evidence of new or worsening liver dysfunction (including clinically significant elevation of liver enzymes and/or symptoms such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) in patients on PREZCOBIX should prompt consideration of interruption or discontinuation of treatment.
Severe Skin Reactions
- During the darunavir clinical development program (n=3063), where darunavir was co-administered with ritonavir 100 mg once or twice daily, severe skin reactions, accompanied by fever and/or elevations of transaminases in some cases, was reported in 0.4% of subjects.
- Stevens-Johnson Syndrome was rarely (less than 0.1%) reported during the clinical development program.
- During post-marketing experience toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis have been reported.
- Discontinue PREZCOBIX immediately if signs or symptoms of severe skin reactions develop.
- These can include but are not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and/or eosinophilia.
- Mild-to-moderate rash was also reported and often occurred within the first four weeks of treatment and resolved with continued dosing.
Effects on Serum Creatinine
- Cobicistat decreases estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function.
- This effect should be considered when interpreting changes in estimated creatinine clearance in patients initiating PREZCOBIX, particularly in patients with medical conditions or receiving drugs needing monitoring with estimated creatinine clearance.
- Prior to initiating therapy with PREZCOBIX, assess estimated creatinine clearance.
- Dosage recommendations are not available for drugs that require dosage adjustments in PREZCOBIX-treated patients with renal impairment.
- Consider alternative medications that do not require dosage adjustments in patients with renal impairment.
- Although cobicistat may cause modest increases in serum creatinine and modest declines in estimated creatinine clearance without affecting renal glomerular function, patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg/dL from baseline should be closely monitored for renal safety.
New Onset or Worsening Renal Impairment when used with Tenofovir Disoproxil Fumarate
- Renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported when cobicistat, a component of PREZCOBIX, was used in an antiretroviral regimen that contained tenofovir DF.
- Co-administration of PREZCOBIX and tenofovir DF is not recommended in patients who have an estimated creatinine clearance below 70 mL/min.
- Document urine glucose and urine protein at baseline and perform routine monitoring of estimated creatinine clearance, urine glucose, and urine protein during treatment when PREZCOBIX is used with tenofovir DF. * Measure serum phosphorus in patients with or at risk for renal impairment when used with tenofovir DF.
- Co-administration of PREZCOBIX and tenofovir DF in combination with concomitant or recent use of a nephrotoxic agent is not recommended.
Risk of Serious Adverse Reactions or Loss of Virologic response due to Drug Interactions
- Initiation of PREZCOBIX, which inhibits CYP3A, in patients receiving medications metabolized by CYP3A, or initiation of medications metabolized by CYP3A in patients already receiving PREZCOBIX may increase plasma concentrations of medications metabolized by CYP3A.
- Initiation of medications that inhibit or induce CYP3A may respectively increase or decrease concentrations of PREZCOBIX.
- Increased concentrations may lead to: clinically significant adverse reactions, potentially leading to severe, life threatening, or fatal events from higher exposures of concomitant medications.
- Clinically significant adverse reactions from higher exposures of PREZCOBIX.
- Decreased antiretroviral concentrations may lead to: loss of therapeutic effect of PREZCOBIX and possible development of resistance.
- See TABLE 2 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations.
- Consider the potential for drug interactions prior to and during PREZCOBIX therapy; review concomitant medications during PREZCOBIX therapy; and monitor for the adverse reactions associated with concomitant medications.
- When used with concomitant medications, PREZCOBIX may result in different drug interactions than those observed or expected with darunavir co-administered with ritonavir.
- Complex or unknown mechanisms of drug interactions preclude extrapolation of drug interactions with darunavir co-administered with ritonavir to certain PREZCOBIX interactions.
Antiretrovirals not Recommended
- PREZCOBIX is not recommended in combination with other antiretroviral drugs that require pharmacokinetic boosting (i.e., another protease inhibitor or elvitegravir) because dosing recommendations for such combinations have not been established and co-administration may result in decreased plasma concentrations of the antiretroviral agents, leading to loss of therapeutic effect and development of resistance.
- PREZCOBIX is not recommended in combination with products containing the individual components of PREZCOBIX (darunavir and cobicistat) or with ritonavir.
Sulfa Allergy
- Darunavir contains a sulfonamide moiety.
- Monitor patients with a known sulfonamide allergy after initiating PREZCOBIX.
- In clinical studies with darunavir co-administered with ritonavir, the incidence and severity of rash were similar in subjects with or without a history of sulfonamide allergy.
Diabetes Mellitus/Hyperglycemia
- New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV infected patients receiving HIV protease inhibitor (PI) therapy.
- Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events.
- In some cases, diabetic ketoacidosis has occurred.
- In those patients who discontinued PI therapy, hyperglycemia persisted in some cases.
- Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between HIV PI therapy and these events have not been established.
Fat Redistribution
- Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy.
- The mechanism and long-term consequences of these events are currently unknown.
- A causal relationship has not been established.
Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including PREZCOBIX.
- During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of antiretroviral treatment.
Hemophilia
- There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with HIV PIs.
- In some patients, additional factor VIII was given.
- In more than half of the reported cases, treatment with HIV PIs was continued or reintroduced if treatment had been discontinued.
- A causal relationship between PI therapy and these episodes has not been established.
|clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- During the darunavir clinical development program, where darunavir was co-administered with ritonavir 100 mg once or twice daily, the most common clinical adverse reactions (incidence greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, nausea, rash, headache, abdominal pain, and vomiting.
- One single arm clinical trial was conducted with darunavir and cobicistat administered as single entities in 313 HIV-infected subjects.
- Adverse reactions evaluated through Week 24 did not differ substantially from those reported in clinical trials with darunavir co-administered with ritonavir.
|drugInteractions===== Potential for PREZCOBIX to affect Other Drugs ====
- When evaluated separately, darunavir and cobicistat both inhibited CYP3A and CYP2D6.
- Cobicistat inhibits the following transporters: p-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3.
- Therefore, co-administration of PREZCOBIX with drugs that are primarily metabolized by CYP3A and/or CYP2D6 or are substrates of P-gp, BCRP, OATP1B1 or OATP1B3 may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and can be associated with adverse events.
Potential for Other Drugs to affect PREZCOBIX
- Darunavir is metabolized by CYP3A.
- Cobicistat is metabolized by CYP3A, and to a minor extent, by CYP2D6.
- Drugs that induce CYP3A activity are expected to increase the clearance of darunavir and cobicistat, resulting in lowered plasma concentrations of darunavir and cobicistat which may lead to loss of therapeutic effect and development of resistance.
- Co-administration of PREZCOBIX and other drugs that inhibit CYP3A may result in increased plasma concentrations of darunavir and cobicistat.
Potentially significant Drug Interactions
- Table 2 provides dosing recommendations for expected clinically relevant interactions with PREZCOBIX.
- These recommendations are based on either drug interaction trials or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of efficacy.
- No drug interaction trials have been performed with PREZCOBIX or with darunavir co-administered with cobicistat as single entities.
- Drug interaction trials have been conducted with darunavir co-administered with ritonavir or with cobicistat alone.
|FDAPregCat=C |useInPregnancyFDA=* PREZCOBIX should be used during pregnancy only if the potential benefit justifies the potential risk.
- No adequate and well-controlled studies have been conducted in pregnant women using darunavir, cobicistat, or PREZCOBIX.
- Animal Data:
- Cobicistat:
- Studies in animals have shown no evidence of teratogenicity or an effect on reproductive function.
- In offspring from rat and rabbit dams treated with cobicistat during pregnancy, there were no toxicologically significant effects on developmental endpoints.
- The exposures at the embryo-fetal No Observed Adverse Effects Levels (NOAELs) in rats and rabbits were respectively 1.4 and 3.3 times higher than the exposure in humans at the recommended daily dose of 150 mg.
- Darunavir:
- Reproduction studies conducted with darunavir showed no embryotoxicity or teratogenicity in mice and rats in the presence or absence of ritonavir as well as in rabbits with darunavir alone.
- In these studies, darunavir exposures (based on AUC) were higher in rats (3-fold), whereas in mice and rabbits, exposures were lower (less than 1-fold) compared to those obtained in humans at the recommended clinical dose of darunavir co-administered with ritonavir.
- In the rat pre- and postnatal development study, a reduction in pup body weight gain was observed with darunavir alone or co-administered with ritonavir during lactation.
- This was due to exposure of pups to drug substances via the milk.
- Sexual development, fertility and mating performance of offspring were not affected by maternal treatment with darunavir alone or co-administered with ritonavir.
- The maximal plasma exposures achieved in rats were approximately 50% of those obtained in humans at the recommended clinical dose boosted with ritonavir.
- In the juvenile toxicity study where rats were directly dosed with darunavir, deaths occurred from post-natal day 5 through 11 at plasma exposure levels ranging from 0.1 to 1.0 of the human exposure levels.
- In a 4 week rat toxicology study, when dosing was initiated on post-natal day 23 (the human equivalent of 2 to 3 years of age), no deaths were observed with a plasma exposure (in combination with ritonavir) of 0.1 of the human plasma exposure levels.
|useInNursing=* The Centers for Disease Control and Prevention recommend that HIV infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV.
- Although it is not known whether darunavir or cobicistat are secreted in human milk, darunavir and cobicistat are secreted into the milk of lactating rats.
- Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, instruct mothers not to breastfeed.
|useInPed=* Safety, effectiveness, and pharmacokinetics of PREZCOBIX in pediatric patients less than 18 years of age have not been established.
- Darunavir, and thus PREZCOBIX is not recommended in pediatric patients below 3 years of age in view of toxicity and mortality observed in juvenile rats dosed with darunavir.
|useInGeri=* Clinical trials of PREZCOBIX did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
- In general, caution should be exercised in the administration and monitoring of PREZCOBIX in elderly patients, reflecting the greater frequency of decreased hepatic function, and of concomitant disease or other drug therapy.
|useInRenalImpair=* A renal impairment trial was not conducted for darunavir co-administered with cobicistat.
- Cobicistat has been shown to decrease estimated creatinine clearance without affecting actual renal glomerular function.
- Dosing recommendations are not available for drugs that require dosage adjustment for renal impairment when used in combination with PREZCOBIX.
|useInHepaticImpair=* No clinical trials were conducted with darunavir co-administered with cobicistat in hepatically impaired subjects and the effect of hepatic impairment on darunavir exposure when co-administered with cobicistat has not been evaluated.
- Based on the recommendations for darunavir co-administered with ritonavir, a dose adjustment for patients with mild or moderate hepatic impairment is not necessary.
- No pharmacokinetic or safety data are available regarding the use of darunavir in subjects with severe hepatic impairment.
- Therefore, PREZCOBIX is not recommended for use in patients with severe hepatic impairment.
|overdose=* Human experience of acute overdose with PREZCOBIX is limited.
- No specific antidote is available for overdose with PREZCOBIX.
- Treatment of overdose with PREZCOBIX consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
- Since both darunavir and cobicistat are highly protein bound, dialysis is unlikely to be beneficial in significant removal of the active substance.
|drugBox=Darunavir ethanolate has the following structural formula:

- Cobicistat has the following structural formula:

|mechAction=* PREZCOBIX is a fixed-dose combination of an HIV-1 antiviral drug, darunavir and a CYP3A inhibitor, cobicistat. |structure=* PREZCOBIX is a fixed-dose combination tablet containing darunavir and cobicistat.
- Darunavir is an inhibitor of the human immunodeficiency virus (HIV-1) protease.
- Cobicistat is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
- PREZCOBIX tablets are for oral administration.
- Each tablet contains darunavir ethanolate equivalent to 800 mg of darunavir and 150 mg of cobicistat.
- The tablets include the following inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose, magnesium stearate, and silicified microcrystalline cellulose.
- The tablets are film-coated with a coating material containing iron oxide black, iron oxide red, polyethylene glycol, polyvinyl alcohol (partially hydrolyzed), talc, and titanium dioxide.
- Darunavir: Darunavir, in the form of darunavir ethanolate, has the following chemical name: [(1S,2R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-carbamic acid (3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl ester monoethanolate.
- Its molecular formula is C27H37N3O7S ∙ C2H5OH and its molecular weight is 593.73.
- Cobicistat:
- Cobicistat is adsorbed onto silicon dioxide.
- The chemical name for cobicistat is 1,3-thiazol-5-ylmethyl[(2R,5R)-5-{[(2S)2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate.
- It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.0.
|PD=* Cardiac Electrophysiology:
- Separate thorough QT trials have been conducted for darunavir co-administered with ritonavir and for cobicistat.
- The effect of darunavir co-administered with cobicistat on the QT interval has not been evaluated.
- Darunavir:
- In an open-label, randomized, placebo- and active-controlled, four-way crossover trial, 40 healthy subjects were administered supratheraputic doses of darunavir 1600 mg and ritonavir 100 mg once daily and darunavir 800 mg and ritonavir 100 mg twice daily (approximately 2 times the recommended darunavir dose) for seven days.
- At the mean maximum darunavir concentration of 6599 ng/mL observed in this trial, the mean increase in QTcF was 2.2 ms with a 90% two-sided confidence interval (CI) of -2.0 to 6.3 ms.
- When evaluating the 2-sided 90% CI on the time-matched mean changes in QTcF versus placebo control, the upper bounds of both darunavir co-administered with ritonavir groups never exceeded the 10 ms boundary.
- In the setting of this trial, darunavir co-administered with ritonavir did not appear to prolong the QTc interval.
- Cobicistat:
- The effect of a single dose of cobicistat 250 mg and 400 mg (approximately 1.7 and 2.7 times the recommended dose) on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) four-period crossover thorough QT trial in 48 healthy subjects.
- In this trial, no significant QTc prolongation effect of cobicistat was detected.
- The dose of 400 mg cobicistat is expected to provide information on a high exposure clinical scenario.
- Prolongation of the PR interval was noted in subjects receiving cobicistat in the same trial.
- The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline-correction was 9.5 (12.1) msec for 250 mg and 20.2 (22.8) msec for 400 mg of cobicistat.
- Effects on Serum Creatinine:
- Cobicistat:
- The effect of cobicistat on serum creatinine was investigated in a trial in subjects with normal renal function (eGFR ≥ 80 mL/min, N=12) and mild-to-moderate renal impairment (eGFR 50–79 mL/min, N=18).
- A statistically significant decrease in the estimated glomerular filtration rate, calculated by Cockcroft-Gault method (eGFRCG) from baseline, was observed after 7 days of treatment with cobicistat 150 mg among subjects with normal renal function (-9.9 ± 13.1 mL/min) and mild-to-moderate renal impairment (-11.9 ± 7.0 mL/min).
- No statistically significant changes in eGFRCG were observed compared to baseline for subjects with normal renal function or mild-to-moderate renal impairment 7 days after cobicistat was discontinued.
- The actual glomerular filtration rate, as determined by the clearance of probe drug iohexol, was not altered from baseline following treatment of cobicistat among subjects with normal renal function and mild-to-moderate renal impairment, indicating that cobicistat inhibits tubular secretion of creatinine, reflected as a reduction in eGFRCG, without affecting the actual glomerular filtration rate.
|PK=* The pharmacokinetics of darunavir co-administered with cobicistat (150 mg) have been evaluated in healthy adult subjects and in HIV-1 infected subjects.
- Darunavir is primarily metabolized by CYP3A. Cobicistat inhibits CYP3A, thereby increasing the plasma concentrations of darunavir.
- Under fed (535 total kcal, 171 kcal from fat, 268 kcal from carbohydrates, 96 kcal from protein) and fasted conditions in healthy subjects, the 90% confidence intervals when comparing darunavir exposure between PREZCOBIX and darunavir 800 mg co-administered with cobicistat 150 mg as single entities were within 80–125%.
- Darunavir exposure when comparing darunavir co-administered with cobicistat (as single entities) to darunavir co-administered with ritonavir was evaluated in a relative bioavailability trial.
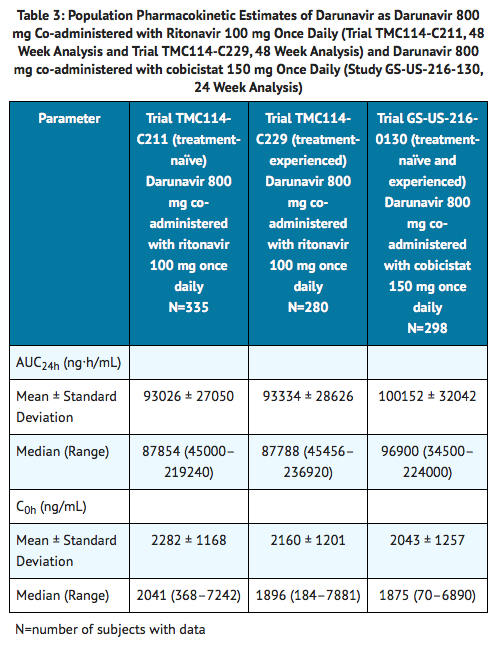
- Table 3 displays the population pharmacokinetic estimates of darunavir after oral administration of darunavir 800 mg co-administered with ritonavir 100 mg once daily (based on sparse sampling in 335 subjects in Trial TMC114-C211 and 280 subjects in Trial TMC114-C229) and darunavir 800 mg co-administered with cobicistat 150 mg once daily administered as single entities (based on sparse sampling in 298 subjects in Trial GS-US-216-0130) to HIV-1 infected subjects.
- Absorption and Bioavailability:
- In healthy subjects, under fed conditions, when single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the maximum plasma concentration was achieved within approximately 4 to 4.5 hours for darunavir and approximately 4 to 5 hours for cobicistat.
- Effects of Food on Oral Absorption:
- When compared to fasted conditions, administration of PREZCOBIX to healthy adult subjects with a high-fat meal (965 total kcal: 129 kcal from protein, 236 kcal from carbohydrates and 600 kcal from fat) resulted in a 70% increase in AUC(0–inf) and a 127% increase in Cmax for darunavir.
- Cobicistat exposures were not affected by food. PREZCOBIX should be taken with food.
- Distribution:
- Darunavir:
- Darunavir is approximately 95% bound to plasma proteins.
- Darunavir binds primarily to plasma alpha 1-acid glycoprotein (AAG).
- Cobicistat:
- Cobicistat is 97–98% bound to human plasma proteins and the mean blood–to-plasma ratio was approximately 0.5.
- Metabolism:
- Darunavir:
- In vitro experiments with human liver microsomes (HLMs) indicate that darunavir primarily undergoes oxidative metabolism.
- Darunavir is extensively metabolized by CYP enzymes, primarily by CYP3A.
- A mass balance trial in healthy subjects showed that after single dose administration of 400 mg 14C-darunavir co-administered with 100 mg ritonavir, the majority of the radioactivity in the plasma was due to darunavir.
- At least 3 oxidative metabolites of darunavir have been identified in humans; all showed activity that was at least 90% less than the activity of darunavir against wild-type HIV-1.
- Cobicistat:
- Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 enzymes and does not undergo glucuronidation.
- Elimination:
- Darunavir:
- A mass balance trial in healthy subjects showed that after single dose administration of 400 mg 14C-darunavir co-administered with 100 mg ritonavir, approximately 79.5% and 13.9% of the administered dose of 14C-darunavir was recovered in the feces and urine, respectively.
- Unchanged darunavir accounted for approximately 41.2% and 7.7% of the administered dose in feces and urine, respectively.
- When single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the terminal elimination half-life of darunavir was approximately 7 hours under fed conditions.
- Cobicistat:
- When single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the terminal elimination half-life of cobicistat was approximately 4 hours under fed conditions.
- With single dose administration of 14C-cobicistat after multiple dosing of cobicistat for six days, the mean percent of the administered dose excreted in feces and urine was 86.2% and 8.2%, respectively.
- Specific Populations:
- Hepatic impairment:
- Darunavir:
- Darunavir is primarily metabolized by the liver.
- The steady-state pharmacokinetic parameters of darunavir were similar after multiple dose co-administration of darunavir 600 mg co-administered with ritonavir 100 mg twice daily to subjects with normal hepatic function (n=16), mild hepatic impairment (Child-Pugh Class A, n=8), and moderate hepatic impairment (Child-Pugh Class B, n=8).
- The effect of severe hepatic impairment on the pharmacokinetics of darunavir has not been evaluated.
- Cobicistat:
- Cobicistat is primarily metabolized by the liver.
- A trial evaluating the pharmacokinetics of cobicistat was performed in non-HIV-1 infected subjects with moderate hepatic impairment.
- No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects.
- The effect of severe hepatic impairment on the pharmacokinetics of cobicistat has not been evaluated.
- Hepatitis B or Hepatitis C Virus Co-infection:
- Darunavir:
- In HIV-infected subjects taking darunavir co-administered with ritonavir, the 48 week analysis of the data from clinical studies in HIV-1 infected subjects indicated that hepatitis B and/or hepatitis C virus co-infection status had no apparent effect on the exposure of darunavir.
- The effect of hepatitis B and/or C virus infection on the pharmacokinetics of PREZCOBIX have not been evaluated.
- Renal Impairment:
- Darunavir:
- Population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV-1 infected subjects with moderate renal impairment taking darunavir co-administered with ritonavir (creatinine clearance between 30–60 mL/min, n=20).
- There are no pharmacokinetic data available in HIV-1 infected patients with severe renal impairment or end stage renal disease taking darunavir co-adminstered with either ritonavir or cobicistat.
- Cobicistat:
- A trial of the pharmacokinetics of cobicistat was performed in non-HIV infected subjects with severe renal impairment (estimated creatinine clearance below 30 mL/min).
- No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with severe renal impairment and healthy subjects.
- Gender:
- Darunavir:
- In HIV-infected subjects taking darunavir co-administered with ritonavir, population pharmacokinetic analysis showed higher mean darunavir exposure in HIV-1 infected females compared to males.
- This difference is not clinically relevant.
- Cobicistat:
- No clinically relevant pharmacokinetic differences have been observed between men and women for cobicistat.
- Race:
- Darunavir:
- Population pharmacokinetic analysis of darunavir in HIV-1 infected subjects taking darunavir co-administered with ritonavir indicated that race had no apparent effect on the exposure to darunavir.
- Cobicistat:
- Population pharmacokinetic analysis of cobicistat in HIV-1 infected subjects indicated that race had no clinically relevant effect on the exposure of cobicistat.
- Geriatric Patients:
- Darunavir:
- In HIV-infected subjects taking darunavir co-administered with ritonavir, population pharmacokinetic analysis showed no considerable differences in darunavir pharmacokinetics for ages 18 to 75 years compared to ages greater than or equal to 65 years (n=12).
- Cobicistat:
- Insufficient data are available to determine whether potential differences exist in the pharmacokinetics of cobicistat in geriatric (65 years of age and older) subjects compared to younger subjects.
- Pediatric Patients:
- The pharmacokinetics of PREZCOBIX in pediatric subjects have not been established.
- Drug Interactions:
- Darunavir is metabolized by CYP3A.
- Cobicistat is metabolized by CYP3A, and to a minor extent, by CYP2D6.
- When evaluated separately, darunavir and cobicistat both inhibited CYP3A and CYP2D6.
- Cobicistat inhibits the following transporters: p-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3. Based on in vitro data, cobicistat is not expected to induce CYP1A2 or CYP2B6 and based on in vivo data, cobicistat is not expected to induce MDR1 or, in general, CYP3A to a clinically significant extent.
- The induction effect of cobicistat on CYP2C9, CYP2C19, or UGT1A1 is unknown, but is expected to be low based on CYP3A in vitro induction data.
|nonClinToxic=* Carcinogenesis, Mutagenesis, Impairment of Fertility:
- Carcinogenesis and Mutagenesis
- Darunavir:
- Darunavir was evaluated for carcinogenic potential by oral gavage administration to mice and rats up to 104 weeks.
- Daily doses of 150, 450 and 1000 mg/kg were administered to mice and doses of 50, 150 and 500 mg/kg were administered to rats.
- A dose-related increase in the incidence of hepatocellular adenomas and carcinomas was observed in males and females of both species and an increase in thyroid follicular cell adenomas was observed in male rats.
- The observed hepatocellular findings in rodents are considered to be of limited relevance to humans.
- Repeated administration of darunavir to rats caused hepatic microsomal enzyme induction and increased thyroid hormone elimination, which predispose rats but not humans, to thyroid neoplasms.
- At the highest tested doses, the systemic exposures to darunavir (based on AUC) were between 0.4- and 0.7-fold (mice) and 0.7- and 1-fold (rats) of exposures observed in humans at the recommended therapeutic doses (darunavir 600 mg co-administered with ritonavir 100 mg twice daily or darunavir 800 mg co-administered with ritonavir 100 mg once daily).
- Darunavir was not mutagenic or genotoxic in a battery of in vitro and in vivo assays including bacterial reverse mutation (Ames), chromosomal aberration in human lymphocytes, and in vivo micronucleus test in mice.
- Cobicistat:
- In a long-term carcinogenicity study in mice, no drug-related increases in tumor incidence were observed at doses up to 50 and 100 mg/kg/day in males and females, respectively.
- Cobicistat exposures at these doses were approximately 7 (male) and 16 (females) times, respectively, the human systemic exposure at the therapeutic daily dose.
- In a long-term carcinogenicity study of cobicistat in rats, an increased incidence of follicular cell adenomas and/or carcinomas in the thyroid gland was observed at doses of 25 and 50 mg/kg/day in males, and at 30 mg/kg/day in females.
- The follicular cell findings are considered to be rat-specific, secondary to hepatic microsomal enzyme induction and thyroid hormone imbalance, and are not relevant for humans.
- At the highest doses tested in the rat carcinogenicity study, systemic exposures were approximately 2 times the human systemic exposure at the therapeutic daily dose.
- Cobicistat was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma or rat micronucleus assays.
- Impairment of Fertility:
- Darunavir:
- No effects on fertility or early embryonic development were observed with darunavir in rats and darunavir has shown no teratogenic potential in mice or rats (in the presence or absence of ritonavir), or in rabbits.
- Cobicistat:
- Cobicistat did not affect fertility in male or female rats at daily exposures (AUC) approximately 4-fold higher than human exposures at the recommended 150 mg daily dose.
- Fertility was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 1.2-fold higher than human exposures at the recommended 150 mg daily dose.
- Animal Toxicology and/or Pharmacology:
- Darunavir:
- In juvenile rats, single doses of darunavir (20 mg/kg to 160 mg/kg at ages 5–11 days) or multiple doses of darunavir (40 mg/kg to 1000 mg/kg at age 12 days) caused mortality.
- The mortalities were associated with convulsions in some of the animals.
- Within this age range exposures in plasma, liver, and brain were dose and age dependent and were considerably greater than those observed in adult rats.
- These findings were attributed to the ontogeny of the CYP450 liver enzymes involved in the metabolism of darunavir and the immaturity of the blood-brain barrier.
- No treatment-related mortalities were noted in juvenile rats after a single dose of darunavir at 1000 mg/kg on day 26 of age or after repeat dosing at 500 mg/kg from day 23 to 50 of age.
- The exposures and toxicity profile in the older animals (day 23 or day 26) were comparable to those observed in adult rats.
- Due to uncertainties regarding the rate of development of the human blood-brain barrier and liver enzymes, do not administer PREZCOBIX in pediatric patients below 3 years of age.
|clinicalStudies=* The efficacy of PREZCOBIX is based on efficacy demonstrated in clinical trials of darunavir co-administered with ritonavir. |howSupplied=* PREZCOBIX (darunavir and cobicistat) tablets, 800/150 mg, are supplied as pink, oval-shaped, film-coated tablets debossed with "800" on one side and "TG" on the other side.
- PREZCOBIX is packaged in bottles of 30 tablets (NDC 59676-575-30).
|storage=* Storage:
- Store at 20–25°C (between 68–77°F); with excursions permitted to 15°–30°C (59°–86°F).
|packLabel===== PRINCIPAL DISPLAY PANEL- 800 MG/150 MG TABLET BOTTLE LABEL ==== NDC 59676-575-30 30 Tablets
PREZCOBIX® (darunavir and cobicistat) Tablets
800mg/150mg
Each tablet contains darunavir ethanolate (equivalent to 800 mg of darunavir) and 150 mg cobicistat.
Rx Only
janssen

|fdaPatientInfo=* Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Instructions for Use:
- Advise patients to take PREZCOBIX with food every day on a regular dosing schedule, as missed doses can result in development of resistance.
- Inform patients not to alter the dose of PREZCOBIX or discontinue therapy with PREZCOBIX without consulting their physician.
- Hepatotoxicity:
- Inform patients that drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) and liver injury, including some fatalities, could potentially occur with PREZCOBIX.
- Advise patients to contact their healthcare provider immediately if signs and symptoms of liver problems develop.
- Severe Skin Reactions:
- Inform patients that skin reactions ranging from mild to severe, including Stevens-Johnson Syndrome, drug rash with eosinophilia and systemic symptoms, and toxic epidermal necrolysis, could potentially occur with PREZCOBIX.
- Advise patients to contact their healthcare provider immediately if signs or symptoms of severe skin reactions develop, including but not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, and/or conjunctivitis.
- Renal Impairment:
- Inform patients that renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported when cobicistat is used in combination with a tenofovir DF-containing regimen.
- Drug Interactions:
- PREZCOBIX may interact with many drugs; therefore, inform patients of the potential serious drug interactions with PREZCOBIX, and that some drugs are contraindicated with PREZCOBIX and other drugs may require dosage adjustment.
- Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John's Wort.
- Instruct patients receiving hormonal contraceptives to use additional or alternative contraceptive (non-hormonal) measures during therapy with PREZCOBIX because no data are available to make recommendations regarding use of hormonal contraceptives and PREZCOBIX.
- Immune Reconstitution Syndrome:
- Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started.
- Fat Redistribution:
- Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy, including PREZCOBIX and that the cause and long-term health effects of these conditions are not known at this time.
- Pregnancy Registry:
- Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to PREZCOBIX.
- Lactation:
- Instruct women with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk.
|alcohol=Alcohol-Darunavir ethanolate and cobicistat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=PREZCOBIX (darunavir and cobicistat) }}