Darunavir ethanolate and cobicistat: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag={{SCh}} |genericName=Darunavir ethanolate and cobicistat |aOrAn=a |drugClass=combination of darunavir, a human immunodeficiency virus (H...") |
No edit summary |
||
| Line 88: | Line 88: | ||
* In more than half of the reported cases, treatment with HIV PIs was continued or reintroduced if treatment had been discontinued. | * In more than half of the reported cases, treatment with HIV PIs was continued or reintroduced if treatment had been discontinued. | ||
* A causal relationship between PI therapy and these episodes has not been established. | * A causal relationship between PI therapy and these episodes has not been established. | ||
|clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
* During the darunavir clinical development program, where darunavir was co-administered with ritonavir 100 mg once or twice daily, the most common clinical adverse reactions (incidence greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, nausea, rash, headache, abdominal pain, and vomiting. | |||
* One single arm clinical trial was conducted with darunavir and cobicistat administered as single entities in 313 HIV-infected subjects. | |||
* Adverse reactions evaluated through Week 24 did not differ substantially from those reported in clinical trials with darunavir co-administered with ritonavir. | |||
|drugInteractions===== Potential for PREZCOBIX to affect Other Drugs ==== | |||
* When evaluated separately, darunavir and cobicistat both inhibited CYP3A and CYP2D6. | |||
* Cobicistat inhibits the following transporters: p-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3. | |||
* Therefore, co-administration of PREZCOBIX with drugs that are primarily metabolized by CYP3A and/or CYP2D6 or are substrates of P-gp, BCRP, OATP1B1 or OATP1B3 may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and can be associated with adverse events. | |||
==== Potential for Other Drugs to affect PREZCOBIX ==== | |||
* Darunavir is metabolized by CYP3A. | |||
* Cobicistat is metabolized by CYP3A, and to a minor extent, by CYP2D6. | |||
* Drugs that induce CYP3A activity are expected to increase the clearance of darunavir and cobicistat, resulting in lowered plasma concentrations of darunavir and cobicistat which may lead to loss of therapeutic effect and development of resistance. | |||
* Co-administration of PREZCOBIX and other drugs that inhibit CYP3A may result in increased plasma concentrations of darunavir and cobicistat. | |||
==== Potentially significant Drug Interactions ==== | |||
* Table 2 provides dosing recommendations for expected clinically relevant interactions with PREZCOBIX. | |||
* These recommendations are based on either drug interaction trials or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of efficacy. | |||
* No drug interaction trials have been performed with PREZCOBIX or with darunavir co-administered with cobicistat as single entities. | |||
* Drug interaction trials have been conducted with darunavir co-administered with ritonavir or with cobicistat alone. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=* PREZCOBIX should be used during pregnancy only if the potential benefit justifies the potential risk. | |||
* No adequate and well-controlled studies have been conducted in pregnant women using darunavir, cobicistat, or PREZCOBIX. | |||
* '''Animal Data''': | |||
* '''Cobicistat''': | |||
:* Studies in animals have shown no evidence of teratogenicity or an effect on reproductive function. | |||
:* In offspring from rat and rabbit dams treated with cobicistat during pregnancy, there were no toxicologically significant effects on developmental endpoints. | |||
:* The exposures at the embryo-fetal No Observed Adverse Effects Levels (NOAELs) in rats and rabbits were respectively 1.4 and 3.3 times higher than the exposure in humans at the recommended daily dose of 150 mg. | |||
* '''Darunavir''': | |||
:* Reproduction studies conducted with darunavir showed no embryotoxicity or teratogenicity in mice and rats in the presence or absence of ritonavir as well as in rabbits with darunavir alone. | |||
:* In these studies, darunavir exposures (based on AUC) were higher in rats (3-fold), whereas in mice and rabbits, exposures were lower (less than 1-fold) compared to those obtained in humans at the recommended clinical dose of darunavir co-administered with ritonavir. | |||
:* In the rat pre- and postnatal development study, a reduction in pup body weight gain was observed with darunavir alone or co-administered with ritonavir during lactation. | |||
:* This was due to exposure of pups to drug substances via the milk. | |||
:* Sexual development, fertility and mating performance of offspring were not affected by maternal treatment with darunavir alone or co-administered with ritonavir. | |||
:* The maximal plasma exposures achieved in rats were approximately 50% of those obtained in humans at the recommended clinical dose boosted with ritonavir. | |||
:* In the juvenile toxicity study where rats were directly dosed with darunavir, deaths occurred from post-natal day 5 through 11 at plasma exposure levels ranging from 0.1 to 1.0 of the human exposure levels. | |||
:* In a 4 week rat toxicology study, when dosing was initiated on post-natal day 23 (the human equivalent of 2 to 3 years of age), no deaths were observed with a plasma exposure (in combination with ritonavir) of 0.1 of the human plasma exposure levels. | |||
|useInNursing=* The Centers for Disease Control and Prevention recommend that HIV infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV. | |||
* Although it is not known whether darunavir or cobicistat are secreted in human milk, darunavir and cobicistat are secreted into the milk of lactating rats. | |||
* Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, instruct mothers not to breastfeed. | |||
|useInPed=* Safety, effectiveness, and pharmacokinetics of PREZCOBIX in pediatric patients less than 18 years of age have not been established. | |||
* Darunavir, and thus PREZCOBIX is not recommended in pediatric patients below 3 years of age in view of toxicity and mortality observed in juvenile rats dosed with darunavir. | |||
|useInGeri=* Clinical trials of PREZCOBIX did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. | |||
* In general, caution should be exercised in the administration and monitoring of PREZCOBIX in elderly patients, reflecting the greater frequency of decreased hepatic function, and of concomitant disease or other drug therapy. | |||
|useInRenalImpair=* A renal impairment trial was not conducted for darunavir co-administered with cobicistat. | |||
* Cobicistat has been shown to decrease estimated creatinine clearance without affecting actual renal glomerular function. | |||
* Dosing recommendations are not available for drugs that require dosage adjustment for renal impairment when used in combination with PREZCOBIX. | |||
|useInHepaticImpair=* No clinical trials were conducted with darunavir co-administered with cobicistat in hepatically impaired subjects and the effect of hepatic impairment on darunavir exposure when co-administered with cobicistat has not been evaluated. | |||
* Based on the recommendations for darunavir co-administered with ritonavir, a dose adjustment for patients with mild or moderate hepatic impairment is not necessary. | |||
* No pharmacokinetic or safety data are available regarding the use of darunavir in subjects with severe hepatic impairment. | |||
* Therefore, PREZCOBIX is not recommended for use in patients with severe hepatic impairment. | |||
|overdose=* Human experience of acute overdose with PREZCOBIX is limited. | |||
* No specific antidote is available for overdose with PREZCOBIX. | |||
* Treatment of overdose with PREZCOBIX consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. | |||
* Since both darunavir and cobicistat are highly protein bound, dialysis is unlikely to be beneficial in significant removal of the active substance. | |||
|mechAction=* PREZCOBIX is a fixed-dose combination of an HIV-1 antiviral drug, darunavir and a CYP3A inhibitor, cobicistat. | |||
|PD=* '''Cardiac Electrophysiology''': | |||
:* Separate thorough QT trials have been conducted for darunavir co-administered with ritonavir and for cobicistat. | |||
* The effect of darunavir co-administered with cobicistat on the QT interval has not been evaluated. | |||
* '''Darunavir''': | |||
:* In an open-label, randomized, placebo- and active-controlled, four-way crossover trial, 40 healthy subjects were administered supratheraputic doses of darunavir 1600 mg and ritonavir 100 mg once daily and darunavir 800 mg and ritonavir 100 mg twice daily (approximately 2 times the recommended darunavir dose) for seven days. | |||
:* At the mean maximum darunavir concentration of 6599 ng/mL observed in this trial, the mean increase in QTcF was 2.2 ms with a 90% two-sided confidence interval (CI) of -2.0 to 6.3 ms. | |||
:* When evaluating the 2-sided 90% CI on the time-matched mean changes in QTcF versus placebo control, the upper bounds of both darunavir co-administered with ritonavir groups never exceeded the 10 ms boundary. | |||
:* In the setting of this trial, darunavir co-administered with ritonavir did not appear to prolong the QTc interval. | |||
* '''Cobicistat''': | |||
:* The effect of a single dose of cobicistat 250 mg and 400 mg (approximately 1.7 and 2.7 times the recommended dose) on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) four-period crossover thorough QT trial in 48 healthy subjects. | |||
:* In this trial, no significant QTc prolongation effect of cobicistat was detected. | |||
:* The dose of 400 mg cobicistat is expected to provide information on a high exposure clinical scenario. | |||
:* Prolongation of the PR interval was noted in subjects receiving cobicistat in the same trial. | |||
:* The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline-correction was 9.5 (12.1) msec for 250 mg and 20.2 (22.8) msec for 400 mg of cobicistat. | |||
* '''Effects on Serum Creatinine''': | |||
* '''Cobicistat''': | |||
:* The effect of cobicistat on serum creatinine was investigated in a trial in subjects with normal renal function (eGFR ≥ 80 mL/min, N=12) and mild-to-moderate renal impairment (eGFR 50–79 mL/min, N=18). | |||
:* A statistically significant decrease in the estimated glomerular filtration rate, calculated by Cockcroft-Gault method (eGFRCG) from baseline, was observed after 7 days of treatment with cobicistat 150 mg among subjects with normal renal function (-9.9 ± 13.1 mL/min) and mild-to-moderate renal impairment (-11.9 ± 7.0 mL/min). | |||
:* No statistically significant changes in eGFRCG were observed compared to baseline for subjects with normal renal function or mild-to-moderate renal impairment 7 days after cobicistat was discontinued. | |||
:* The actual glomerular filtration rate, as determined by the clearance of probe drug iohexol, was not altered from baseline following treatment of cobicistat among subjects with normal renal function and mild-to-moderate renal impairment, indicating that cobicistat inhibits tubular secretion of creatinine, reflected as a reduction in eGFRCG, without affecting the actual glomerular filtration rate. | |||
|PK=* The pharmacokinetics of darunavir co-administered with cobicistat (150 mg) have been evaluated in healthy adult subjects and in HIV-1 infected subjects. | |||
* Darunavir is primarily metabolized by CYP3A. Cobicistat inhibits CYP3A, thereby increasing the plasma concentrations of darunavir. | |||
* Under fed (535 total kcal, 171 kcal from fat, 268 kcal from carbohydrates, 96 kcal from protein) and fasted conditions in healthy subjects, the 90% confidence intervals when comparing darunavir exposure between PREZCOBIX and darunavir 800 mg co-administered with cobicistat 150 mg as single entities were within 80–125%. | |||
* Darunavir exposure when comparing darunavir co-administered with cobicistat (as single entities) to darunavir co-administered with ritonavir was evaluated in a relative bioavailability trial. | |||
* Table 3 displays the population pharmacokinetic estimates of darunavir after oral administration of darunavir 800 mg co-administered with ritonavir 100 mg once daily (based on sparse sampling in 335 subjects in Trial TMC114-C211 and 280 subjects in Trial TMC114-C229) and darunavir 800 mg co-administered with cobicistat 150 mg once daily administered as single entities (based on sparse sampling in 298 subjects in Trial GS-US-216-0130) to HIV-1 infected subjects. | |||
[[File:Table 3 DARUNAVIR.png|400px|thumb|none|This image is provided by the National Library of Medicine]] | |||
* '''Absorption and Bioavailability''': | |||
:* In healthy subjects, under fed conditions, when single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the maximum plasma concentration was achieved within approximately 4 to 4.5 hours for darunavir and approximately 4 to 5 hours for cobicistat. | |||
* '''Effects of Food on Oral Absorption''': | |||
:* When compared to fasted conditions, administration of PREZCOBIX to healthy adult subjects with a high-fat meal (965 total kcal: 129 kcal from protein, 236 kcal from carbohydrates and 600 kcal from fat) resulted in a 70% increase in AUC(0–inf) and a 127% increase in Cmax for darunavir. | |||
:* Cobicistat exposures were not affected by food. PREZCOBIX should be taken with food. | |||
* '''Distribution''': | |||
* '''Darunavir''': | |||
:* Darunavir is approximately 95% bound to plasma proteins. | |||
:* Darunavir binds primarily to plasma alpha 1-acid glycoprotein (AAG). | |||
* '''Cobicistat''': | |||
:* Cobicistat is 97–98% bound to human plasma proteins and the mean blood–to-plasma ratio was approximately 0.5. | |||
* '''Metabolism''': | |||
* '''Darunavir''': | |||
:* In vitro experiments with human liver microsomes (HLMs) indicate that darunavir primarily undergoes oxidative metabolism. | |||
* Darunavir is extensively metabolized by CYP enzymes, primarily by CYP3A. | |||
* A mass balance trial in healthy subjects showed that after single dose administration of 400 mg 14C-darunavir co-administered with 100 mg ritonavir, the majority of the radioactivity in the plasma was due to darunavir. | |||
* At least 3 oxidative metabolites of darunavir have been identified in humans; all showed activity that was at least 90% less than the activity of darunavir against wild-type HIV-1. | |||
* '''Cobicistat''': | |||
:* Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 enzymes and does not undergo glucuronidation. | |||
* '''Elimination''': | |||
* Darunavir: | |||
:* A mass balance trial in healthy subjects showed that after single dose administration of 400 mg 14C-darunavir co-administered with 100 mg ritonavir, approximately 79.5% and 13.9% of the administered dose of 14C-darunavir was recovered in the feces and urine, respectively. | |||
:* Unchanged darunavir accounted for approximately 41.2% and 7.7% of the administered dose in feces and urine, respectively. | |||
:* When single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the terminal elimination half-life of darunavir was approximately 7 hours under fed conditions. | |||
* '''Cobicistat''': | |||
:* When single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the terminal elimination half-life of cobicistat was approximately 4 hours under fed conditions. | |||
:* With single dose administration of 14C-cobicistat after multiple dosing of cobicistat for six days, the mean percent of the administered dose excreted in feces and urine was 86.2% and 8.2%, respectively. | |||
* '''Specific Populations''': | |||
* '''Hepatic impairment''': | |||
* '''Darunavir''': | |||
:* Darunavir is primarily metabolized by the liver. | |||
:* The steady-state pharmacokinetic parameters of darunavir were similar after multiple dose co-administration of darunavir 600 mg co-administered with ritonavir 100 mg twice daily to subjects with normal hepatic function (n=16), mild hepatic impairment (Child-Pugh Class A, n=8), and moderate hepatic impairment (Child-Pugh Class B, n=8). | |||
:* The effect of severe hepatic impairment on the pharmacokinetics of darunavir has not been evaluated. | |||
* '''Cobicistat''': | |||
:* Cobicistat is primarily metabolized by the liver. | |||
:* A trial evaluating the pharmacokinetics of cobicistat was performed in non-HIV-1 infected subjects with moderate hepatic impairment. | |||
:* No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects. | |||
:* The effect of severe hepatic impairment on the pharmacokinetics of cobicistat has not been evaluated. | |||
* '''Hepatitis B or Hepatitis C Virus Co-infection''': | |||
* '''Darunavir''': | |||
:* In HIV-infected subjects taking darunavir co-administered with ritonavir, the 48 week analysis of the data from clinical studies in HIV-1 infected subjects indicated that hepatitis B and/or hepatitis C virus co-infection status had no apparent effect on the exposure of darunavir. | |||
:* The effect of hepatitis B and/or C virus infection on the pharmacokinetics of PREZCOBIX have not been evaluated. | |||
* '''Renal Impairment''': | |||
* '''Darunavir''': | |||
:* Population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV-1 infected subjects with moderate renal impairment taking darunavir co-administered with ritonavir (creatinine clearance between 30–60 mL/min, n=20). | |||
:* There are no pharmacokinetic data available in HIV-1 infected patients with severe renal impairment or end stage renal disease taking darunavir co-adminstered with either ritonavir or cobicistat. | |||
* '''Cobicistat''': | |||
:* A trial of the pharmacokinetics of cobicistat was performed in non-HIV infected subjects with severe renal impairment (estimated creatinine clearance below 30 mL/min). | |||
:* No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with severe renal impairment and healthy subjects. | |||
* '''Gender''': | |||
* '''Darunavir''': | |||
:* In HIV-infected subjects taking darunavir co-administered with ritonavir, population pharmacokinetic analysis showed higher mean darunavir exposure in HIV-1 infected females compared to males. | |||
:* This difference is not clinically relevant. | |||
* '''Cobicistat''': | |||
:* No clinically relevant pharmacokinetic differences have been observed between men and women for cobicistat. | |||
* Race: | |||
* '''Darunavir''': | |||
:* Population pharmacokinetic analysis of darunavir in HIV-1 infected subjects taking darunavir co-administered with ritonavir indicated that race had no apparent effect on the exposure to darunavir. | |||
* '''Cobicistat''': | |||
:* Population pharmacokinetic analysis of cobicistat in HIV-1 infected subjects indicated that race had no clinically relevant effect on the exposure of cobicistat. | |||
* '''Geriatric Patients''': | |||
* '''Darunavir''': | |||
:* In HIV-infected subjects taking darunavir co-administered with ritonavir, population pharmacokinetic analysis showed no considerable differences in darunavir pharmacokinetics for ages 18 to 75 years compared to ages greater than or equal to 65 years (n=12). | |||
* '''Cobicistat''': | |||
:* Insufficient data are available to determine whether potential differences exist in the pharmacokinetics of cobicistat in geriatric (65 years of age and older) subjects compared to younger subjects. | |||
* '''Pediatric Patients''': | |||
:* The pharmacokinetics of PREZCOBIX in pediatric subjects have not been established. | |||
* '''Drug Interactions''': | |||
:* Darunavir is metabolized by CYP3A. | |||
:* Cobicistat is metabolized by CYP3A, and to a minor extent, by CYP2D6. | |||
:* When evaluated separately, darunavir and cobicistat both inhibited CYP3A and CYP2D6. | |||
:* Cobicistat inhibits the following transporters: p-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3. Based on in vitro data, cobicistat is not expected to induce CYP1A2 or CYP2B6 and based on in vivo data, cobicistat is not expected to induce MDR1 or, in general, CYP3A to a clinically significant extent. | |||
:* The induction effect of cobicistat on CYP2C9, CYP2C19, or UGT1A1 is unknown, but is expected to be low based on CYP3A in vitro induction data. | |||
|alcohol=Alcohol-Darunavir ethanolate and cobicistat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Darunavir ethanolate and cobicistat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 20:16, 10 March 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Shivani Chaparala M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Darunavir ethanolate and cobicistat is a combination of darunavir, a human immunodeficiency virus (HIV-1) protease inhibitor and cobicistat, a CYP3A inhibitor that is FDA approved for the treatment of HIV-1 infection in adult patients.. Common adverse reactions include Darunavir: diarrhea, nausea, rash, headache, abdominal pain, and vomiting..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- PREZCOBIX® is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection in treatment-naïve and treatment-experienced adults with no darunavir resistance-associated substitutions. Recommended dosage: One tablet taken once daily with food. Tablets: 800 mg of darunavir and 150 mg of cobicistat.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Darunavir ethanolate and cobicistat in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Darunavir ethanolate and cobicistat in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Darunavir ethanolate and cobicistat FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Darunavir ethanolate and cobicistat in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Darunavir ethanolate and cobicistat in pediatric patients.
Contraindications
There is limited information regarding Darunavir ethanolate and cobicistat Contraindications in the drug label.
Warnings
Hepatotoxicity
- During the darunavir clinical development program (N=3063), where darunavir was co-administered with ritonavir 100 mg once or twice daily, drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) was reported in 0.5% of subjects.
- Patients with pre-existing liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities including severe hepatic adverse reactions.
- Post-marketing cases of liver injury, including some fatalities, have also been reported with darunavir co-administered with ritonavir.
- These have generally occurred in patients with advanced HIV-1 disease taking multiple concomitant medications, having co-morbidities including hepatitis B or C co-infection, and/or developing immune reconstitution syndrome.
- A causal relationship with darunavir co-administered with ritonavir has not been established.
- Appropriate laboratory testing should be conducted prior to initiating therapy with PREZCOBIX and patients should be monitored during treatment.
- Increased AST/ALT monitoring should be considered in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases, especially during the first several months of PREZCOBIX treatment.
- Evidence of new or worsening liver dysfunction (including clinically significant elevation of liver enzymes and/or symptoms such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) in patients on PREZCOBIX should prompt consideration of interruption or discontinuation of treatment.
Severe Skin Reactions
- During the darunavir clinical development program (n=3063), where darunavir was co-administered with ritonavir 100 mg once or twice daily, severe skin reactions, accompanied by fever and/or elevations of transaminases in some cases, was reported in 0.4% of subjects.
- Stevens-Johnson Syndrome was rarely (less than 0.1%) reported during the clinical development program.
- During post-marketing experience toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis have been reported.
- Discontinue PREZCOBIX immediately if signs or symptoms of severe skin reactions develop.
- These can include but are not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis and/or eosinophilia.
- Mild-to-moderate rash was also reported and often occurred within the first four weeks of treatment and resolved with continued dosing.
Effects on Serum Creatinine
- Cobicistat decreases estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting actual renal glomerular function.
- This effect should be considered when interpreting changes in estimated creatinine clearance in patients initiating PREZCOBIX, particularly in patients with medical conditions or receiving drugs needing monitoring with estimated creatinine clearance.
- Prior to initiating therapy with PREZCOBIX, assess estimated creatinine clearance.
- Dosage recommendations are not available for drugs that require dosage adjustments in PREZCOBIX-treated patients with renal impairment.
- Consider alternative medications that do not require dosage adjustments in patients with renal impairment.
- Although cobicistat may cause modest increases in serum creatinine and modest declines in estimated creatinine clearance without affecting renal glomerular function, patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg/dL from baseline should be closely monitored for renal safety.
New Onset or Worsening Renal Impairment when used with Tenofovir Disoproxil Fumarate
- Renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported when cobicistat, a component of PREZCOBIX, was used in an antiretroviral regimen that contained tenofovir DF.
- Co-administration of PREZCOBIX and tenofovir DF is not recommended in patients who have an estimated creatinine clearance below 70 mL/min.
- Document urine glucose and urine protein at baseline and perform routine monitoring of estimated creatinine clearance, urine glucose, and urine protein during treatment when PREZCOBIX is used with tenofovir DF. * Measure serum phosphorus in patients with or at risk for renal impairment when used with tenofovir DF.
- Co-administration of PREZCOBIX and tenofovir DF in combination with concomitant or recent use of a nephrotoxic agent is not recommended.
Risk of Serious Adverse Reactions or Loss of Virologic response due to Drug Interactions
- Initiation of PREZCOBIX, which inhibits CYP3A, in patients receiving medications metabolized by CYP3A, or initiation of medications metabolized by CYP3A in patients already receiving PREZCOBIX may increase plasma concentrations of medications metabolized by CYP3A.
- Initiation of medications that inhibit or induce CYP3A may respectively increase or decrease concentrations of PREZCOBIX.
- Increased concentrations may lead to: clinically significant adverse reactions, potentially leading to severe, life threatening, or fatal events from higher exposures of concomitant medications.
- Clinically significant adverse reactions from higher exposures of PREZCOBIX.
- Decreased antiretroviral concentrations may lead to: loss of therapeutic effect of PREZCOBIX and possible development of resistance.
- See TABLE 2 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations.
- Consider the potential for drug interactions prior to and during PREZCOBIX therapy; review concomitant medications during PREZCOBIX therapy; and monitor for the adverse reactions associated with concomitant medications.
- When used with concomitant medications, PREZCOBIX may result in different drug interactions than those observed or expected with darunavir co-administered with ritonavir.
- Complex or unknown mechanisms of drug interactions preclude extrapolation of drug interactions with darunavir co-administered with ritonavir to certain PREZCOBIX interactions.
Antiretrovirals not Recommended
- PREZCOBIX is not recommended in combination with other antiretroviral drugs that require pharmacokinetic boosting (i.e., another protease inhibitor or elvitegravir) because dosing recommendations for such combinations have not been established and co-administration may result in decreased plasma concentrations of the antiretroviral agents, leading to loss of therapeutic effect and development of resistance.
- PREZCOBIX is not recommended in combination with products containing the individual components of PREZCOBIX (darunavir and cobicistat) or with ritonavir.
Sulfa Allergy
- Darunavir contains a sulfonamide moiety.
- Monitor patients with a known sulfonamide allergy after initiating PREZCOBIX.
- In clinical studies with darunavir co-administered with ritonavir, the incidence and severity of rash were similar in subjects with or without a history of sulfonamide allergy.
Diabetes Mellitus/Hyperglycemia
- New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV infected patients receiving HIV protease inhibitor (PI) therapy.
- Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events.
- In some cases, diabetic ketoacidosis has occurred.
- In those patients who discontinued PI therapy, hyperglycemia persisted in some cases.
- Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between HIV PI therapy and these events have not been established.
Fat Redistribution
- Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy.
- The mechanism and long-term consequences of these events are currently unknown.
- A causal relationship has not been established.
Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including PREZCOBIX.
- During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of antiretroviral treatment.
Hemophilia
- There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with HIV PIs.
- In some patients, additional factor VIII was given.
- In more than half of the reported cases, treatment with HIV PIs was continued or reintroduced if treatment had been discontinued.
- A causal relationship between PI therapy and these episodes has not been established.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
- During the darunavir clinical development program, where darunavir was co-administered with ritonavir 100 mg once or twice daily, the most common clinical adverse reactions (incidence greater than or equal to 5%) of at least moderate intensity (greater than or equal to Grade 2) were diarrhea, nausea, rash, headache, abdominal pain, and vomiting.
- One single arm clinical trial was conducted with darunavir and cobicistat administered as single entities in 313 HIV-infected subjects.
- Adverse reactions evaluated through Week 24 did not differ substantially from those reported in clinical trials with darunavir co-administered with ritonavir.
Postmarketing Experience
There is limited information regarding Darunavir ethanolate and cobicistat Postmarketing Experience in the drug label.
Drug Interactions
Potential for PREZCOBIX to affect Other Drugs
- When evaluated separately, darunavir and cobicistat both inhibited CYP3A and CYP2D6.
- Cobicistat inhibits the following transporters: p-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3.
- Therefore, co-administration of PREZCOBIX with drugs that are primarily metabolized by CYP3A and/or CYP2D6 or are substrates of P-gp, BCRP, OATP1B1 or OATP1B3 may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and can be associated with adverse events.
Potential for Other Drugs to affect PREZCOBIX
- Darunavir is metabolized by CYP3A.
- Cobicistat is metabolized by CYP3A, and to a minor extent, by CYP2D6.
- Drugs that induce CYP3A activity are expected to increase the clearance of darunavir and cobicistat, resulting in lowered plasma concentrations of darunavir and cobicistat which may lead to loss of therapeutic effect and development of resistance.
- Co-administration of PREZCOBIX and other drugs that inhibit CYP3A may result in increased plasma concentrations of darunavir and cobicistat.
Potentially significant Drug Interactions
- Table 2 provides dosing recommendations for expected clinically relevant interactions with PREZCOBIX.
- These recommendations are based on either drug interaction trials or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of efficacy.
- No drug interaction trials have been performed with PREZCOBIX or with darunavir co-administered with cobicistat as single entities.
- Drug interaction trials have been conducted with darunavir co-administered with ritonavir or with cobicistat alone.
Use in Specific Populations
Pregnancy
- PREZCOBIX should be used during pregnancy only if the potential benefit justifies the potential risk.
- No adequate and well-controlled studies have been conducted in pregnant women using darunavir, cobicistat, or PREZCOBIX.
- Animal Data:
- Cobicistat:
- Studies in animals have shown no evidence of teratogenicity or an effect on reproductive function.
- In offspring from rat and rabbit dams treated with cobicistat during pregnancy, there were no toxicologically significant effects on developmental endpoints.
- The exposures at the embryo-fetal No Observed Adverse Effects Levels (NOAELs) in rats and rabbits were respectively 1.4 and 3.3 times higher than the exposure in humans at the recommended daily dose of 150 mg.
- Darunavir:
- Reproduction studies conducted with darunavir showed no embryotoxicity or teratogenicity in mice and rats in the presence or absence of ritonavir as well as in rabbits with darunavir alone.
- In these studies, darunavir exposures (based on AUC) were higher in rats (3-fold), whereas in mice and rabbits, exposures were lower (less than 1-fold) compared to those obtained in humans at the recommended clinical dose of darunavir co-administered with ritonavir.
- In the rat pre- and postnatal development study, a reduction in pup body weight gain was observed with darunavir alone or co-administered with ritonavir during lactation.
- This was due to exposure of pups to drug substances via the milk.
- Sexual development, fertility and mating performance of offspring were not affected by maternal treatment with darunavir alone or co-administered with ritonavir.
- The maximal plasma exposures achieved in rats were approximately 50% of those obtained in humans at the recommended clinical dose boosted with ritonavir.
- In the juvenile toxicity study where rats were directly dosed with darunavir, deaths occurred from post-natal day 5 through 11 at plasma exposure levels ranging from 0.1 to 1.0 of the human exposure levels.
- In a 4 week rat toxicology study, when dosing was initiated on post-natal day 23 (the human equivalent of 2 to 3 years of age), no deaths were observed with a plasma exposure (in combination with ritonavir) of 0.1 of the human plasma exposure levels.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Darunavir ethanolate and cobicistat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Darunavir ethanolate and cobicistat during labor and delivery.
Nursing Mothers
- The Centers for Disease Control and Prevention recommend that HIV infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV.
- Although it is not known whether darunavir or cobicistat are secreted in human milk, darunavir and cobicistat are secreted into the milk of lactating rats.
- Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, instruct mothers not to breastfeed.
Pediatric Use
- Safety, effectiveness, and pharmacokinetics of PREZCOBIX in pediatric patients less than 18 years of age have not been established.
- Darunavir, and thus PREZCOBIX is not recommended in pediatric patients below 3 years of age in view of toxicity and mortality observed in juvenile rats dosed with darunavir.
Geriatic Use
- Clinical trials of PREZCOBIX did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
- In general, caution should be exercised in the administration and monitoring of PREZCOBIX in elderly patients, reflecting the greater frequency of decreased hepatic function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Darunavir ethanolate and cobicistat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Darunavir ethanolate and cobicistat with respect to specific racial populations.
Renal Impairment
- A renal impairment trial was not conducted for darunavir co-administered with cobicistat.
- Cobicistat has been shown to decrease estimated creatinine clearance without affecting actual renal glomerular function.
- Dosing recommendations are not available for drugs that require dosage adjustment for renal impairment when used in combination with PREZCOBIX.
Hepatic Impairment
- No clinical trials were conducted with darunavir co-administered with cobicistat in hepatically impaired subjects and the effect of hepatic impairment on darunavir exposure when co-administered with cobicistat has not been evaluated.
- Based on the recommendations for darunavir co-administered with ritonavir, a dose adjustment for patients with mild or moderate hepatic impairment is not necessary.
- No pharmacokinetic or safety data are available regarding the use of darunavir in subjects with severe hepatic impairment.
- Therefore, PREZCOBIX is not recommended for use in patients with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Darunavir ethanolate and cobicistat in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Darunavir ethanolate and cobicistat in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Darunavir ethanolate and cobicistat Administration in the drug label.
Monitoring
There is limited information regarding Darunavir ethanolate and cobicistat Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Darunavir ethanolate and cobicistat and IV administrations.
Overdosage
- Human experience of acute overdose with PREZCOBIX is limited.
- No specific antidote is available for overdose with PREZCOBIX.
- Treatment of overdose with PREZCOBIX consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
- Since both darunavir and cobicistat are highly protein bound, dialysis is unlikely to be beneficial in significant removal of the active substance.
Pharmacology
There is limited information regarding Darunavir ethanolate and cobicistat Pharmacology in the drug label.
Mechanism of Action
- PREZCOBIX is a fixed-dose combination of an HIV-1 antiviral drug, darunavir and a CYP3A inhibitor, cobicistat.
Structure
There is limited information regarding Darunavir ethanolate and cobicistat Structure in the drug label.
Pharmacodynamics
- Cardiac Electrophysiology:
- Separate thorough QT trials have been conducted for darunavir co-administered with ritonavir and for cobicistat.
- The effect of darunavir co-administered with cobicistat on the QT interval has not been evaluated.
- Darunavir:
- In an open-label, randomized, placebo- and active-controlled, four-way crossover trial, 40 healthy subjects were administered supratheraputic doses of darunavir 1600 mg and ritonavir 100 mg once daily and darunavir 800 mg and ritonavir 100 mg twice daily (approximately 2 times the recommended darunavir dose) for seven days.
- At the mean maximum darunavir concentration of 6599 ng/mL observed in this trial, the mean increase in QTcF was 2.2 ms with a 90% two-sided confidence interval (CI) of -2.0 to 6.3 ms.
- When evaluating the 2-sided 90% CI on the time-matched mean changes in QTcF versus placebo control, the upper bounds of both darunavir co-administered with ritonavir groups never exceeded the 10 ms boundary.
- In the setting of this trial, darunavir co-administered with ritonavir did not appear to prolong the QTc interval.
- Cobicistat:
- The effect of a single dose of cobicistat 250 mg and 400 mg (approximately 1.7 and 2.7 times the recommended dose) on QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) four-period crossover thorough QT trial in 48 healthy subjects.
- In this trial, no significant QTc prolongation effect of cobicistat was detected.
- The dose of 400 mg cobicistat is expected to provide information on a high exposure clinical scenario.
- Prolongation of the PR interval was noted in subjects receiving cobicistat in the same trial.
- The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline-correction was 9.5 (12.1) msec for 250 mg and 20.2 (22.8) msec for 400 mg of cobicistat.
- Effects on Serum Creatinine:
- Cobicistat:
- The effect of cobicistat on serum creatinine was investigated in a trial in subjects with normal renal function (eGFR ≥ 80 mL/min, N=12) and mild-to-moderate renal impairment (eGFR 50–79 mL/min, N=18).
- A statistically significant decrease in the estimated glomerular filtration rate, calculated by Cockcroft-Gault method (eGFRCG) from baseline, was observed after 7 days of treatment with cobicistat 150 mg among subjects with normal renal function (-9.9 ± 13.1 mL/min) and mild-to-moderate renal impairment (-11.9 ± 7.0 mL/min).
- No statistically significant changes in eGFRCG were observed compared to baseline for subjects with normal renal function or mild-to-moderate renal impairment 7 days after cobicistat was discontinued.
- The actual glomerular filtration rate, as determined by the clearance of probe drug iohexol, was not altered from baseline following treatment of cobicistat among subjects with normal renal function and mild-to-moderate renal impairment, indicating that cobicistat inhibits tubular secretion of creatinine, reflected as a reduction in eGFRCG, without affecting the actual glomerular filtration rate.
Pharmacokinetics
- The pharmacokinetics of darunavir co-administered with cobicistat (150 mg) have been evaluated in healthy adult subjects and in HIV-1 infected subjects.
- Darunavir is primarily metabolized by CYP3A. Cobicistat inhibits CYP3A, thereby increasing the plasma concentrations of darunavir.
- Under fed (535 total kcal, 171 kcal from fat, 268 kcal from carbohydrates, 96 kcal from protein) and fasted conditions in healthy subjects, the 90% confidence intervals when comparing darunavir exposure between PREZCOBIX and darunavir 800 mg co-administered with cobicistat 150 mg as single entities were within 80–125%.
- Darunavir exposure when comparing darunavir co-administered with cobicistat (as single entities) to darunavir co-administered with ritonavir was evaluated in a relative bioavailability trial.
- Table 3 displays the population pharmacokinetic estimates of darunavir after oral administration of darunavir 800 mg co-administered with ritonavir 100 mg once daily (based on sparse sampling in 335 subjects in Trial TMC114-C211 and 280 subjects in Trial TMC114-C229) and darunavir 800 mg co-administered with cobicistat 150 mg once daily administered as single entities (based on sparse sampling in 298 subjects in Trial GS-US-216-0130) to HIV-1 infected subjects.
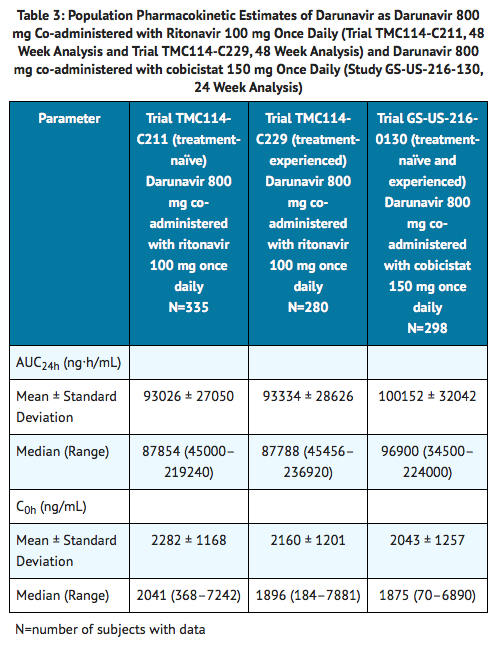
- Absorption and Bioavailability:
- In healthy subjects, under fed conditions, when single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the maximum plasma concentration was achieved within approximately 4 to 4.5 hours for darunavir and approximately 4 to 5 hours for cobicistat.
- Effects of Food on Oral Absorption:
- When compared to fasted conditions, administration of PREZCOBIX to healthy adult subjects with a high-fat meal (965 total kcal: 129 kcal from protein, 236 kcal from carbohydrates and 600 kcal from fat) resulted in a 70% increase in AUC(0–inf) and a 127% increase in Cmax for darunavir.
- Cobicistat exposures were not affected by food. PREZCOBIX should be taken with food.
- Distribution:
- Darunavir:
- Darunavir is approximately 95% bound to plasma proteins.
- Darunavir binds primarily to plasma alpha 1-acid glycoprotein (AAG).
- Cobicistat:
- Cobicistat is 97–98% bound to human plasma proteins and the mean blood–to-plasma ratio was approximately 0.5.
- Metabolism:
- Darunavir:
- In vitro experiments with human liver microsomes (HLMs) indicate that darunavir primarily undergoes oxidative metabolism.
- Darunavir is extensively metabolized by CYP enzymes, primarily by CYP3A.
- A mass balance trial in healthy subjects showed that after single dose administration of 400 mg 14C-darunavir co-administered with 100 mg ritonavir, the majority of the radioactivity in the plasma was due to darunavir.
- At least 3 oxidative metabolites of darunavir have been identified in humans; all showed activity that was at least 90% less than the activity of darunavir against wild-type HIV-1.
- Cobicistat:
- Cobicistat is metabolized by CYP3A and to a minor extent by CYP2D6 enzymes and does not undergo glucuronidation.
- Elimination:
- Darunavir:
- A mass balance trial in healthy subjects showed that after single dose administration of 400 mg 14C-darunavir co-administered with 100 mg ritonavir, approximately 79.5% and 13.9% of the administered dose of 14C-darunavir was recovered in the feces and urine, respectively.
- Unchanged darunavir accounted for approximately 41.2% and 7.7% of the administered dose in feces and urine, respectively.
- When single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the terminal elimination half-life of darunavir was approximately 7 hours under fed conditions.
- Cobicistat:
- When single doses of the darunavir and cobicistat fixed dose combination tablet were administered, the terminal elimination half-life of cobicistat was approximately 4 hours under fed conditions.
- With single dose administration of 14C-cobicistat after multiple dosing of cobicistat for six days, the mean percent of the administered dose excreted in feces and urine was 86.2% and 8.2%, respectively.
- Specific Populations:
- Hepatic impairment:
- Darunavir:
- Darunavir is primarily metabolized by the liver.
- The steady-state pharmacokinetic parameters of darunavir were similar after multiple dose co-administration of darunavir 600 mg co-administered with ritonavir 100 mg twice daily to subjects with normal hepatic function (n=16), mild hepatic impairment (Child-Pugh Class A, n=8), and moderate hepatic impairment (Child-Pugh Class B, n=8).
- The effect of severe hepatic impairment on the pharmacokinetics of darunavir has not been evaluated.
- Cobicistat:
- Cobicistat is primarily metabolized by the liver.
- A trial evaluating the pharmacokinetics of cobicistat was performed in non-HIV-1 infected subjects with moderate hepatic impairment.
- No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects.
- The effect of severe hepatic impairment on the pharmacokinetics of cobicistat has not been evaluated.
- Hepatitis B or Hepatitis C Virus Co-infection:
- Darunavir:
- In HIV-infected subjects taking darunavir co-administered with ritonavir, the 48 week analysis of the data from clinical studies in HIV-1 infected subjects indicated that hepatitis B and/or hepatitis C virus co-infection status had no apparent effect on the exposure of darunavir.
- The effect of hepatitis B and/or C virus infection on the pharmacokinetics of PREZCOBIX have not been evaluated.
- Renal Impairment:
- Darunavir:
- Population pharmacokinetic analysis showed that the pharmacokinetics of darunavir were not significantly affected in HIV-1 infected subjects with moderate renal impairment taking darunavir co-administered with ritonavir (creatinine clearance between 30–60 mL/min, n=20).
- There are no pharmacokinetic data available in HIV-1 infected patients with severe renal impairment or end stage renal disease taking darunavir co-adminstered with either ritonavir or cobicistat.
- Cobicistat:
- A trial of the pharmacokinetics of cobicistat was performed in non-HIV infected subjects with severe renal impairment (estimated creatinine clearance below 30 mL/min).
- No clinically relevant differences in cobicistat pharmacokinetics were observed between subjects with severe renal impairment and healthy subjects.
- Gender:
- Darunavir:
- In HIV-infected subjects taking darunavir co-administered with ritonavir, population pharmacokinetic analysis showed higher mean darunavir exposure in HIV-1 infected females compared to males.
- This difference is not clinically relevant.
- Cobicistat:
- No clinically relevant pharmacokinetic differences have been observed between men and women for cobicistat.
- Race:
- Darunavir:
- Population pharmacokinetic analysis of darunavir in HIV-1 infected subjects taking darunavir co-administered with ritonavir indicated that race had no apparent effect on the exposure to darunavir.
- Cobicistat:
- Population pharmacokinetic analysis of cobicistat in HIV-1 infected subjects indicated that race had no clinically relevant effect on the exposure of cobicistat.
- Geriatric Patients:
- Darunavir:
- In HIV-infected subjects taking darunavir co-administered with ritonavir, population pharmacokinetic analysis showed no considerable differences in darunavir pharmacokinetics for ages 18 to 75 years compared to ages greater than or equal to 65 years (n=12).
- Cobicistat:
- Insufficient data are available to determine whether potential differences exist in the pharmacokinetics of cobicistat in geriatric (65 years of age and older) subjects compared to younger subjects.
- Pediatric Patients:
- The pharmacokinetics of PREZCOBIX in pediatric subjects have not been established.
- Drug Interactions:
- Darunavir is metabolized by CYP3A.
- Cobicistat is metabolized by CYP3A, and to a minor extent, by CYP2D6.
- When evaluated separately, darunavir and cobicistat both inhibited CYP3A and CYP2D6.
- Cobicistat inhibits the following transporters: p-glycoprotein (P-gp), BCRP, OATP1B1 and OATP1B3. Based on in vitro data, cobicistat is not expected to induce CYP1A2 or CYP2B6 and based on in vivo data, cobicistat is not expected to induce MDR1 or, in general, CYP3A to a clinically significant extent.
- The induction effect of cobicistat on CYP2C9, CYP2C19, or UGT1A1 is unknown, but is expected to be low based on CYP3A in vitro induction data.
Nonclinical Toxicology
There is limited information regarding Darunavir ethanolate and cobicistat Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Darunavir ethanolate and cobicistat Clinical Studies in the drug label.
How Supplied
There is limited information regarding Darunavir ethanolate and cobicistat How Supplied in the drug label.
Storage
There is limited information regarding Darunavir ethanolate and cobicistat Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Darunavir ethanolate and cobicistat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Darunavir ethanolate and cobicistat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Darunavir ethanolate and cobicistat Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Darunavir ethanolate and cobicistat interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Darunavir ethanolate and cobicistat Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Darunavir ethanolate and cobicistat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.