Danazol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
ConditionName:
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Danazol is a {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Endometriosis
- Danazol capsules are indicated for the treatment of endometriosis amenable to hormonal management.
- Dosing Information
- In moderate to severe disease, or in patients infertile due to endometriosis, a starting dose of 800 mg given in two divided doses is recommended. Amenorrhea and rapid response to painful symptoms is best achieved at this dosage level. Gradual downward titration to a dose sufficient to maintain amenorrhea may be considered depending upon patient response. For mild cases, an initial daily dose of 200 mg to 400 mg given in two divided doses is recommended and may be adjusted depending on patient response. Therapy should begin during menstruation. Otherwise, appropriate tests should be performed to ensure that the patient is not pregnant while on therapy with danazol (see CONTRAINDICATIONS and WARNINGS). It is essential that therapy continue uninterrupted for 3 to 6 months but may be extended to 9 months if necessary. After termination of therapy, if symptoms recur, treatment can be reinstituted.
Fibrocystic Breast Disease
- Most cases of symptomatic fibrocystic breast disease may be treated by simple measures (e.g., padded brassieres and analgesics).
- In infrequent patients, symptoms of pain and tenderness may be severe enough to warrant treatment by suppression of ovarian function. Danazol capsules are usually effective in decreasing nodularity, pain, and tenderness. It should be stressed to the patient that this treatment is not innocuous in that it involves considerable alterations of hormone levels and that recurrence of symptoms is very common after cessation of therapy.
- Dosing Information
- The total daily dosage of danazol for fibrocystic breast disease ranges from 100 mg to 400 mg given in two divided doses depending upon patient response. Therapy should begin during menstruation. Otherwise, appropriate tests should be performed to ensure that the patient is not pregnant while on therapy with danazol. A nonhormonal method of contraception is recommended when danazol is administered at this dose, since ovulation may not be suppressed.
- In most instances, breast pain and tenderness are significantly relieved by the first month and eliminated in 2 to 3 months. Usually elimination of nodularity requires 4 to 6 months of uninterrupted therapy. Regular menstrual patterns, irregular menstrual patterns, and amenorrhea each occur in approximately one-third of patients treated with 100 mg of danazol. Irregular menstrual patterns and amenorrhea are observed more frequently with higher doses. Clinical studies have demonstrated that 50% of patients may show evidence of recurrence of symptoms within one year. In this event, treatment may be reinstated.
Hereditary Angioedema
- Danazol capsules are indicated for the prevention of attacks of angioedema of all types (cutaneous, abdominal, laryngeal) in males and females.
- Dosing Information
- The dosage requirements for continuous treatment of hereditary angioedema with danazol should be individualized on the basis of the clinical response of the patient. It is recommended that the patient be started on 200 mg, two or three times a day. After a favorable initial response is obtained in terms of prevention of episodes of edematous attacks, the proper continuing dosage should be determined by decreasing the dosage by 50% or less at intervals of one to three months or longer if frequency of attacks prior to treatment dictates. If an attack occurs, the daily dosage may be increased by up to 200 mg. During the dose adjusting phase, close monitoring of the patient's response is indicated, particularly if the patient has a history of airway involvement.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Danazol in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Danazol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Danazol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Danazol in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Danazol in pediatric patients.
Contraindications
- Condition1
Warnings
|
ConditionName:
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Danazol in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Danazol in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Danazol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Danazol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Danazol with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Danazol with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Danazol with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Danazol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Danazol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Danazol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Danazol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Danazol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Danazol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Danazol in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Danazol in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Danazol in the drug label.
Pharmacology
Mechanism of Action
Structure
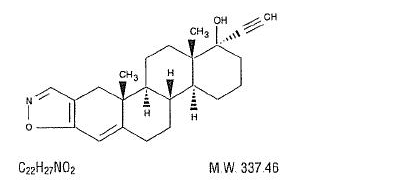
Danazol is a synthetic steroid derived from ethisterone. It is a white to pale yellow crystalline powder, practically insoluble or insoluble in water, and sparingly soluble in alcohol. Chemically, danazol is 17α-Pregna-2, 4-dien-20-yno [2, 3-d]-isoxazol-17-ol. It has the following structural formula:
Danazol capsules for oral administration contain 50 mg, 100 mg, or 200 mg danazol.
Inactive Ingredients: anhydrous lactose, lactose monohydrate, magnesium stearate, pregelatinized starch, sodium lauryl sulfate, talc. Capsule shells for 200 mg danazol contain D&C Yellow #10, FD&C Red #40, D&C Red #28, gelatin, and titanium dioxide. Capsule shells for 50 mg and 100 mg danazol contain D&C Yellow # 10, FD&C Red # 40, gelatin, and titanium dioxide. The capsule imprinting ink contains: shellac glaze in ethanol, iron oxide black, n-butyl alcohol, propylene glycol, ethanol, methanol, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, and D&C Yellow No. 10 Aluminum Lake.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Danazol in the drug label.
Pharmacokinetics
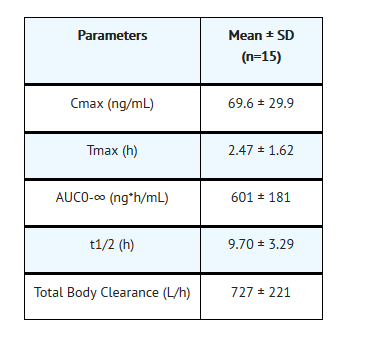
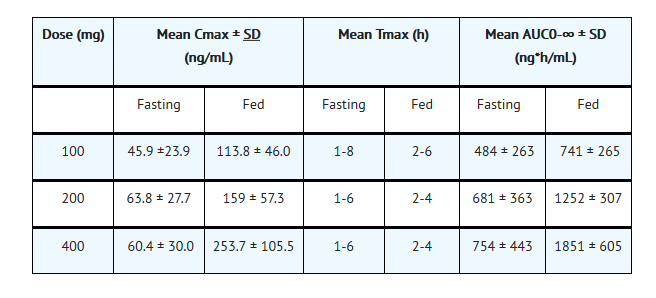
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Danazol in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Danazol in the drug label.
How Supplied
Storage
There is limited information regarding Danazol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Danazol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

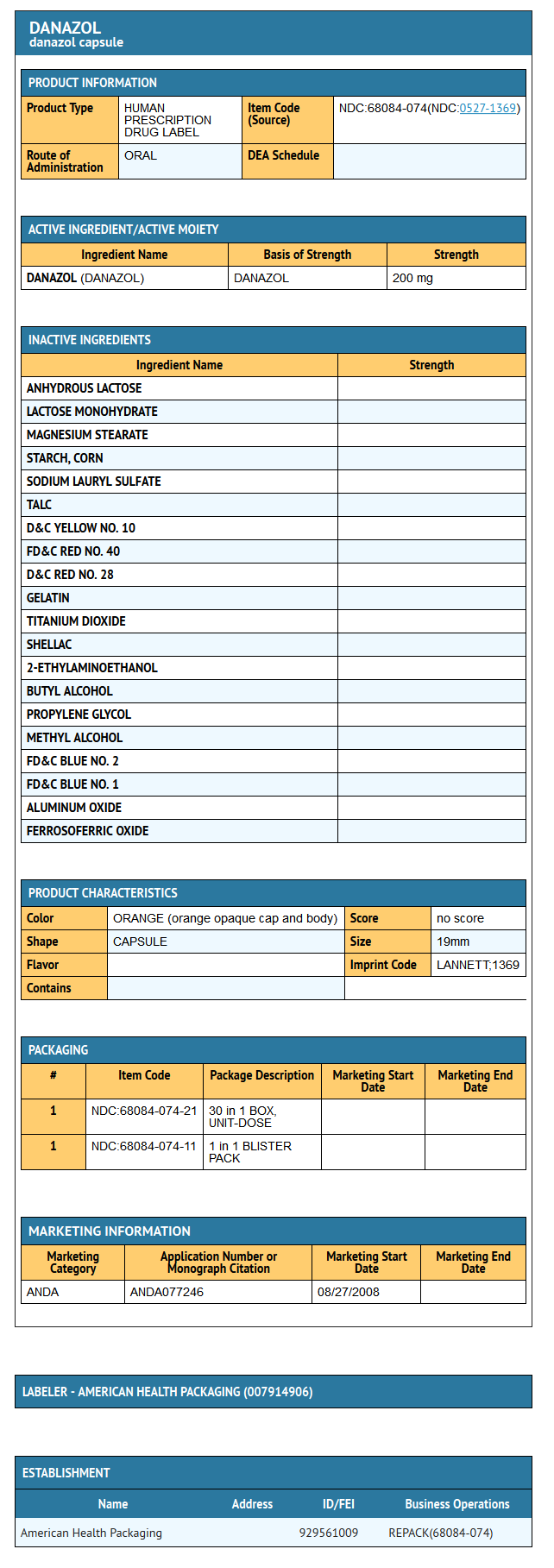
{{#ask: Label Page::Danazol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Danazol in the drug label.
Precautions with Alcohol
- Alcohol-Danazol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Danazol
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Danazol |Label Name=Danazol11.png
}}
{{#subobject:
|Label Page=Danazol |Label Name=Danazol11.png
}}