Cytochrome: Difference between revisions
No edit summary |
(No difference)
|
Revision as of 17:52, 3 June 2009
|
WikiDoc Resources for Cytochrome |
|
Articles |
|---|
|
Most recent articles on Cytochrome |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cytochrome at Clinical Trials.gov Clinical Trials on Cytochrome at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cytochrome
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cytochrome Discussion groups on Cytochrome Patient Handouts on Cytochrome Directions to Hospitals Treating Cytochrome Risk calculators and risk factors for Cytochrome
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cytochrome |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
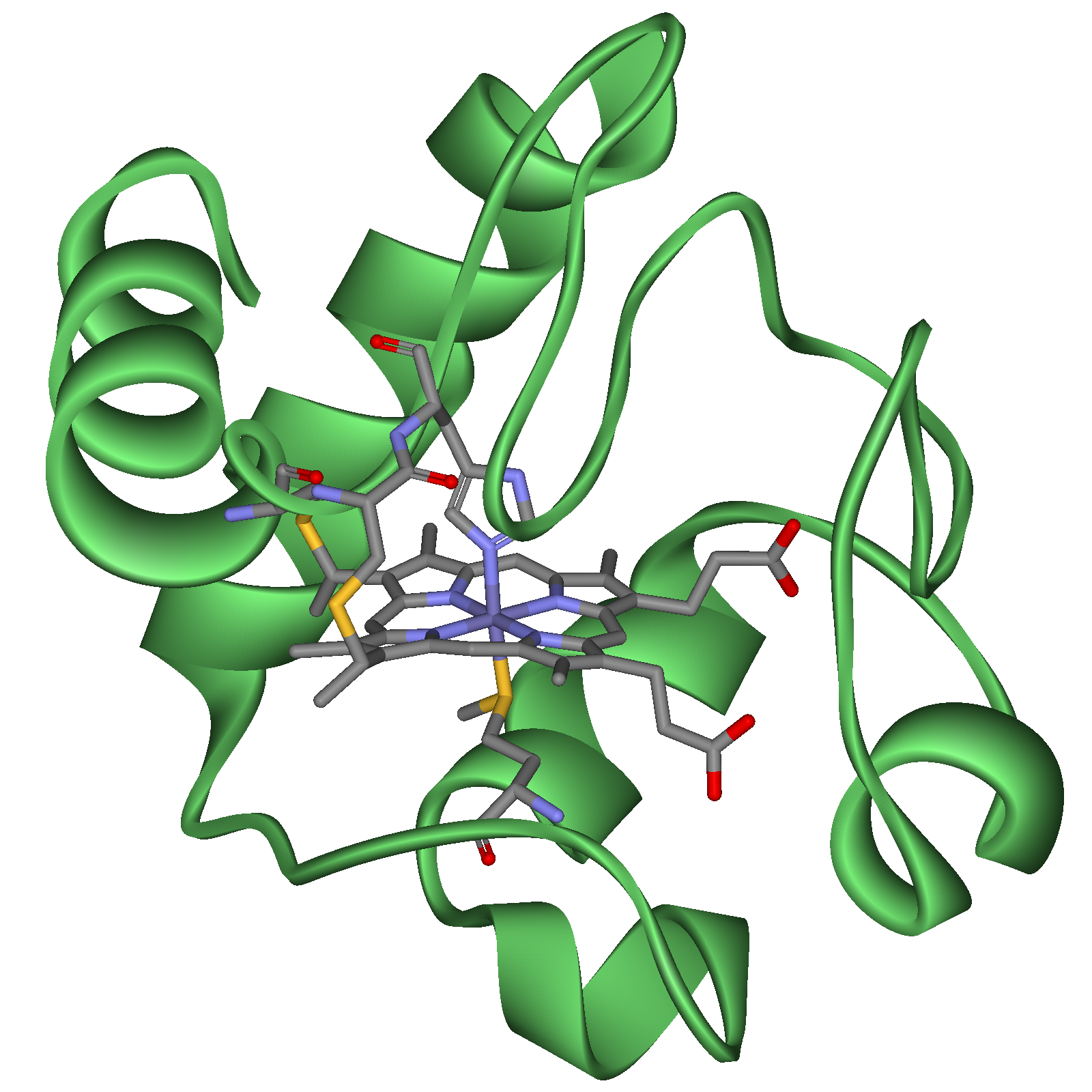
Cytochromes are generally membrane-bound hemoproteins that contain heme groups and carry out electron transport.
They are either found as monomeric proteins (i.e. cytochrome c) or as subunits of bigger enzymatic complexes that catalyze redox reactions. They are found in the mitochondrial inner membrane and endoplasmic reticulum of eukaryotes, in the chloroplasts of plants, in photosynthetic microorganisms, and in bacteria.
Structure and function
The heme group is a highly conjugated ring system (which means its electrons are very mobile) surrounding a metal ion, which readily interconverts between the oxidation states. For many cytochromes the metal ion present is that of iron, which interconverts between Fe2+ (reduced) and Fe3+ (oxidized) states (electron-transfer processes) or between Fe2+ (reduced) and Fe3+ (formal, oxidized) states (oxidative processes). Cytochromes are thus capable of performing oxidation and reduction. Because the cytochromes (as well as other complexes) are held within membranes in an organized way, the redox reactions are carried out in the proper sequence for maximum efficiency.
In the process of oxidative phosphorylation, which is the principal energy-generating process undertaken by organisms which need oxygen to survive, other membrane-bound and soluble complexes and cofactors are involved in the chain of redox reactions, with the additional net effect that protons (H+) are transported across the mitochondrial inner membrane. The resulting transmembrane proton gradient [(protonmotive force)] is used to generate ATP, which is the universal chemical energy currency of life. ATP is consumed to drive cellular processes that require energy (such as synthesis of macromolecules, active transport of molecules across the membrane, and assembly of flagella).
Types
Several kinds of cytochrome exist and can be distinguished by spectroscopy, exact structure of the heme group, inhibitor sensitivity, and reduction potential.
Three types of cytochrome are distinguished by their prosthetic groups:
| Type | prosthetic group |
| Cytochrome a | heme a |
| Cytochrome b | heme b |
| Cytochrome d | tetrapyrrolic chelate of iron[1] |
The definition of cytochrome c is not defined in terms of the heme group.[2] There is no "cytochrome e", but there is a cytochrome f, which is often considered a type of cytochrome c.[3]
In mitochondria and chloroplasts, these cytochromes are often combined in electron transport and related metabolic pathways:
| Cytochromes | Combination |
| a and a3 | Cytochrome c oxidase ("Complex IV") |
| b and c1 | Coenzyme Q - cytochrome c reductase ("Complex III") |
| b6 and f | Plastoquinol—plastocyanin reductase |
A completely distinct family of cytochromes are known as the cytochrome P450 oxidases, so named for the characteristic Soret peak formed by absorbance of light at wavelengths near 450 nm when the heme iron is reduced (with sodium dithionite) and complexed to carbon monoxide. These enzymes are primarily involved in steroidogenesis and detoxification.
References
- ↑ Cytochrome+d at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Cytochrome+c+Group at the US National Library of Medicine Medical Subject Headings (MeSH).
- ↑ Template:EMedicineDictionary
External links
- Scripps Database of Metalloproteins
- Cytochromes at the US National Library of Medicine Medical Subject Headings (MeSH)
Template:SIB de:Cytochrom it:Citocromi he:ציטוכרום mk:Цитохром