Cyclosporiasis laboratory findings
|
Cyclosporiasis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cyclosporiasis laboratory findings On the Web |
|
American Roentgen Ray Society Images of Cyclosporiasis laboratory findings |
|
Risk calculators and risk factors for Cyclosporiasis laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Laboratory Findings
Stool Examination
Wet Mount
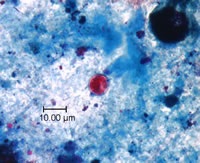
In bright-field microscopy using differential interference contrast (DIC), oocysts appear as refractile spheres (8 to 10 μm) with a distinct oocyst wall, but may be confused with other objects. Under UV fluorescence microscopy, the oocyst wall autofluoresces. An intense blue fluorescence is obtained with the preferred UV excitation filter set (330 to 365 nm). If this filter set is not available, a less intense green fluorescence can be obtained with blue excitation (450 to 490 nm). Other objects, however, can also autofluoresce. A fluorescence microscope is required and this procedure does not provide a stained slide that can be archived.Both DIC and UV fluorescence microscopy are efficient and reliable approaches for identification of this coccidian. Objects found by UV microscopy should always be checked under DIC and vice versa.
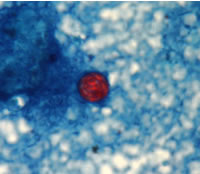
Modified acid-fast stain
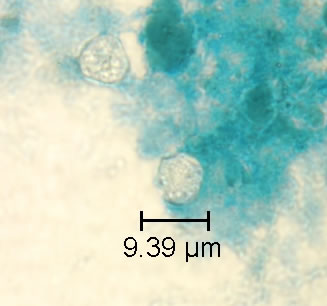 File:Acid fats stain 3.jpg
A blue-green background, or contrasting counterstain, of fecal debris allows the oocysts to stand out. The oocysts are variably stained: some will stain light pink to deep purple, while others may be unstained. The oocysts (8 to 10 μm) may not be perfectly round; some may appear collapsed or distorted on one side. They may contain granules and/or have a wrinkled oocyst wall appearance (characteristics that distinguish oocysts from acid-fast artifacts). This staining method is the easiest and most practical, and provides a stained slide that can be archived. Misdiagnosis can result, however, due to the variability in staining and confusion with artifacts.
File:Acid fats stain 3.jpg
A blue-green background, or contrasting counterstain, of fecal debris allows the oocysts to stand out. The oocysts are variably stained: some will stain light pink to deep purple, while others may be unstained. The oocysts (8 to 10 μm) may not be perfectly round; some may appear collapsed or distorted on one side. They may contain granules and/or have a wrinkled oocyst wall appearance (characteristics that distinguish oocysts from acid-fast artifacts). This staining method is the easiest and most practical, and provides a stained slide that can be archived. Misdiagnosis can result, however, due to the variability in staining and confusion with artifacts.
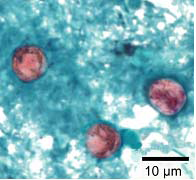
Safranin stain
Oocysts stain uniformly, red to reddish-orange. This uniform staining decreases the risk of misdiagnosis. However, this technique requires heating, therefore additional equipment is necessary (e.g., microwave oven).
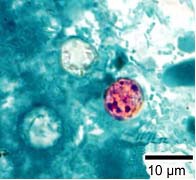
Trichrome stain
Oocysts may be detected, but should not be confirmed, by this method. Because trichrome stain is the routine staining technique for stool specimens in most laboratories, laboratorians should be familiar with the appearance of Cyclospora stained with trichrome in order to detect oocysts during routine ex-aminations. However, this staining method is inadequate for definitive diagnosis because all oocysts will appear unstained. Oocysts appear as clear, round, and somewhat wrinkled spheres (8 to 10 μm). The diagnostic techniques listed above should be used to confirm Cyclospora when the presence of this coccidian is suspected in a trichrome stained smear.
External Link
http://www.dpd.cdc.gov/dpdx/HTML/PDF_Files/cyclospora_benchaid.pdf