Colorectal cancer diagnostic study of choice: Difference between revisions
| Line 136: | Line 136: | ||
[[Image:Colorectal cancer staging.png|thumb|300px|left|T category based on local extent of tumor]] | [[Image:Colorectal cancer staging.png|thumb|300px|left|T category based on local extent of tumor]] | ||
< | <br> | ||
* | * | ||
Revision as of 20:10, 22 January 2019
|
Colorectal cancer Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Colorectal cancer diagnostic study of choice On the Web |
|
American Roentgen Ray Society Images of Colorectal cancer diagnostic study of choice |
|
Risk calculators and risk factors for Colorectal cancer diagnostic study of choice |
To view the staging of familial adenomatous polyposis (FAP), click here
To view the staging of hereditary nonpolyposis colorectal cancer (HNPCC), click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Saarah T. Alkhairy, M.D.; Elliot B. Tapper, M.D.
Overview
Diagnostic Study of Choice
Study of choice
[Name of the investigation] is the gold standard test for the diagnosis of [disease name].
OR
The following result of [gold standard test] is confirmatory of [disease name]:
- [Result 1]
- [Result 2]
OR
[Name of the investigation] must be performed when:
- The patient presents with [symptom/sign 1], [symptom/sign 2], and [symptom/sign 3].
- A [name of test] is positive for [sign 1], [sign 2], and [sign 3] in the patient.
OR
[Name of the investigation] is the gold standard test for the diagnosis of [disease name].
OR
The diagnostic study of choice for [disease name] is [name of the investigation].
OR
There is no single diagnostic study of choice for the diagnosis of [disease name].
OR
There is no single diagnostic study of choice for the diagnosis of [disease name], but [disease name] can be diagnosed based on [name of the investigation 1] and [name of the investigation 2].
OR
[Disease name] is primarily diagnosed based on the clinical presentation.
OR
Investigations:
- Among the patients who present with clinical signs of [disease name], the [investigation name] is the most specific test for the diagnosis.
- Among the patients who present with clinical signs of [disease name], the [investigation name] is the most sensitive test for diagnosis.
- Among the patients who present with clinical signs of [disease name], the [investigation name] is the most efficient test for diagnosis.
The comparison of various diagnostic studies for [disease name]
| Test | Sensitivity | Specificity |
|---|---|---|
| Test 1 | ...% | ...% |
| Test 2 | ...% | ...% |
[Name of test with higher sensitivity and specificity] is the preferred investigation based on the sensitivity and specificity
Diagnostic results
The following finding(s) on performing [investigation name] is(are) confirmatory for [disease name]:
- [Finding 1]
- [Finding 2]
Sequence of Diagnostic Studies
The [name of investigation] must be performed when:
- The patient presented with symptoms/signs 1, 2, and 3 as the first step of diagnosis.
- A positive [test] is detected in the patient, to confirm the diagnosis.
OR
The various investigations must be performed in the following order:
- [Initial investigation]
- [2nd investigation]
Name of Diagnostic Criteria
It is recommended that you include the criteria in a table. Make sure you always cite the source of the content and whether the table has been adapted from another source.
[Disease name] is primarily diagnosed based on clinical presentation. There are no established criteria for the diagnosis of [disease name].
OR
There is no single diagnostic study of choice for [disease name], though [disease name] may be diagnosed based on [name of criteria] established by [...].
OR
The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met: [criterion 1], [criterion 2], [criterion 3], and [criterion 4].
OR
The diagnosis of [disease name] is based on the [criteria name] criteria, which includes [criterion 1], [criterion 2], and [criterion 3].
OR
[Disease name] may be diagnosed at any time if one or more of the following criteria are met:
- Criteria 1
- Criteria 2
- Criteria 3
OR
IF there are clear, established diagnostic criteria
The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met: [criterion 1], [criterion 2], [criterion 3], and [criterion 4].
OR
The diagnosis of [disease name] is based on the [criteria name] criteria, which include [criterion 1], [criterion 2], and [criterion 3].
OR
The diagnosis of [disease name] is based on the [definition name] definition, which includes [criterion 1], [criterion 2], and [criterion 3].
OR
There are no established criteria for the diagnosis of [disease name].
Colorectal Cancer Staging
- Staging of colorectal cancer is calculated after the diagnosis has been established in order to assess treatment and prognosis.
- The most recent revision (2017) of the tumor, node, metastasis staging system (TNM) proposed by the American Joint Committe on Cancer and the Union for International Cancer Control is widely used.
- This staging system depends on 3 main factors including the size of the tumor (T), the degree of lymph node involvement (N), and the presence or absence of distant metastasis (M).
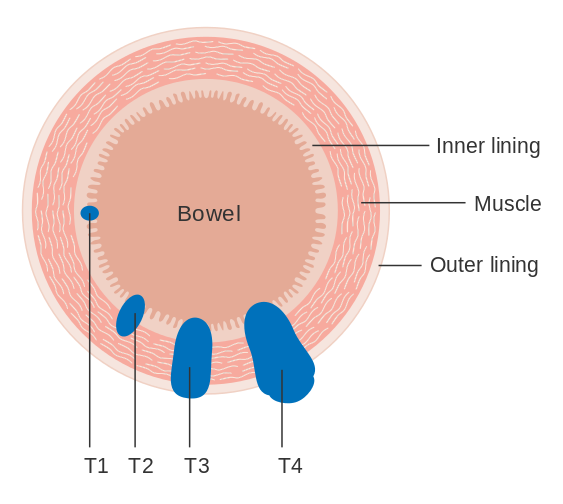
- Definitive staging can only be done after surgery has been performed and pathology reports have been reviewed
The most common staging system is the TNM classification (tumors/nodes/metastases) system. The TNM classification system assigns a number[1].
The table below displays which TNM values represent which stage.
| Stage | TNM Value |
| 0 | Tis, N0, M0 |
| I | T1, N0, M0; T2, N0, M0 |
| IIA | T3, N0, M0 |
| IIB | T4, N0, M0 |
| IIIA | T1, N1, M0; T2, N1, M0 |
| IIIB | T3, N1, M0; T4, N1, M0 |
| IIIC | Any T, N2, M0 |
| IV | Any T, Any N, M1 |
References
- ↑ Wittekind, Ch; Sobin, L. H. (2002). TNM classification of malignant tumours. New York: Wiley-Liss. ISBN 0-471-22288-7.