Coagulation factor VIIa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Ammu Susheela, M.D. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SERIOUS THROMBOTIC ADVERSE EVENTS ASSOCIATED WITH THE USE OF NovoSeven RT OUTSIDE LABELED INDICATIONS
See full prescribing information for complete Boxed Warning.
* Arterial and venous thrombotic and thromboembolic events following administration of NovoSeven have been reported during postmarketing surveillance.
|
Overview
Coagulation factor VIIa is a human blood coagulation factor that is FDA approved for the treatment of bleeding episodes and peri-operative management in adults and children with hemophilia A or B with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions, with or without antibodies to platelets, bleeding episodes and peri-operative management in adults with acquired hemophilia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
General
- Dosing information
- Coagulation factor VIIa should be administered to patients only under the supervision of a physician experienced in the treatment of bleeding disorders.
- Evaluation of hemostasis should be used to determine the effectiveness of coagulation factor VIIa and to provide a basis for modification of the coagulation factor VIIa treatment schedule.
- Coagulation parameters do not necessarily correlate with or predict the effectiveness of coagulation factor VIIa.
- Hemophilia A or B with Inhibitors
- Dosing for Treatment of Acute Bleeding Episodes
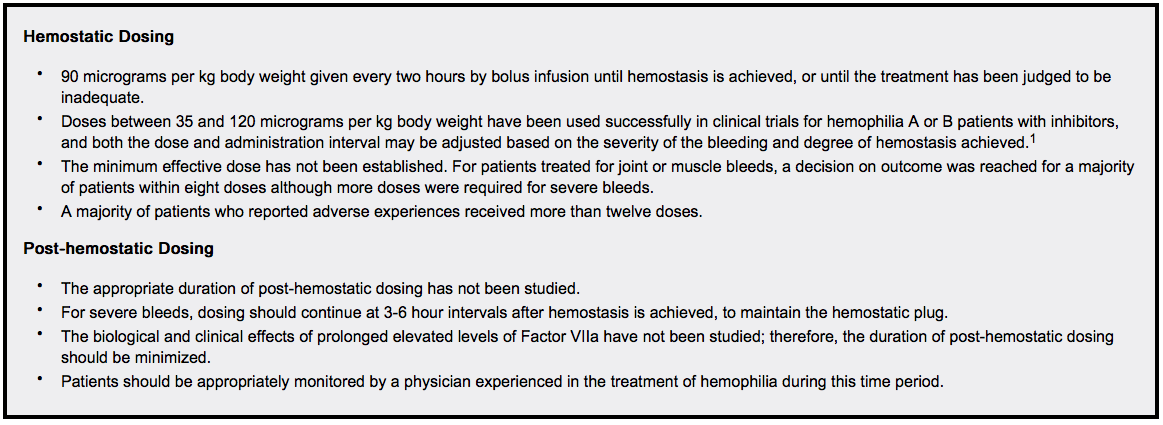
- Dosing for Surgical Interventions

Congenital Factor VII Deficiency
- Dosing information
- Dose and frequency of injections should be adjusted to each individual.
- The recommended dose range for treatment of bleeding episodes or for prevention of bleeding in surgical interventions or invasive procedures in congenital Factor VII deficient patients is 15-30 micrograms per kg body weight every 4-6 hours until hemostasis is achieved.
- Effective treatment has been achieved with doses as low as 10 micrograms per kg body weight.The minimum effective dose has not been determined.
Acquired Hemophilia
- Dosing information
- The recommended dose range for the treatment of patients with acquired hemophilia is 70-90 micrograms per kg body weight repeated every 2-3 hours until hemostasis is achieved.
- The minimum effective dose in acquired hemophilia has not been determined.
- The majority of the effective outcomes were observed with treatment in the recommended dose range. The largest number of treatments with any single dose was 90 micrograms per kg body weight; of the 15 treated, 10 (67%) were effective and 2 (13%) were partially effective.
Reconstitution
- Dosing information
- Patients should follow the specific reconstitution procedures provided by their physician. Instructions for Use are provided in the FDA-approved patient labeling accompanying coagulation factor VIIa. The procedures below are general guidelines for the preparation and reconstitution of coagulation factor VIIa. For questions regarding reconstitution, please contact Novo Nordisk at 1-877-NOVO-777.
- Calculate the coagulation factor VIIa dosage and select the appropriate coagulation factor VIIa package provided with either 1 histidine diluent vial or 1 pre-filled histidine diluent syringe.
- Reconstitute only with the histidine diluent provided with coagulation factor VIIa.
Coagulation factor VIIa package containing 1 vial of coagulation factor VIIa powder and 1 vial of histidine diluent:
- Always use aseptic technique.
- Bring coagulation factor VIIa (white, lyophilized powder) and the specified volume of histidine (diluent) to room temperature, but not above 37° C (98.6° F). The specified volume of diluent corresponding to the amount of coagulation factor VIIa is as follows:
- 1 mg (1000 micrograms) vial + 1.1 mL Histidine diluent
- 2 mg (2000 micrograms) vial + 2.1 mL Histidine diluent
- 5 mg (5000 micrograms) vial + 5.2 mL Histidine diluent
- 8 mg (8000 micrograms) vial + 8.1 mL Histidine diluent
- Remove caps from the coagulation factor VIIa vials to expose the central portion of the rubber stopper. Cleanse the rubber stoppers with an alcohol swab and allow to dry prior to use.
- Draw back the plunger of a sterile syringe (attached to sterile needle) and admit air into the syringe. It is recommended to use syringe needles of gauge size 20-26.
- Insert the needle of the syringe into the Histidine diluent vial. Inject air into the vial and withdraw the quantity required for reconstitution.
- Insert the syringe needle containing the diluent into the coagulation factor VIIa vial through the center of the rubber stopper, aiming the needle against the side so that the stream of liquid runs down the vial wall (the coagulation factor VIIa vial does not contain a vacuum). Do not inject the diluent directly on the coagulation factor VIIa powder.
- Gently swirl the vial until all the material is dissolved. The reconstituted solution is a clear, colorless solution which may be stored either at room temperature or refrigerated for up to 3 hours after reconstitution. After reconstitution with the specified volume of diluent, each vial contains approximately 1 mg per mL coagulation factor VIIa (1000 micrograms per mL).
coagulation factor VIIa package containing 1 vial of coagulation factor VIIa powder and 1 pre-filled histidine diluent syringe with vial adapter for needleless reconstitution:
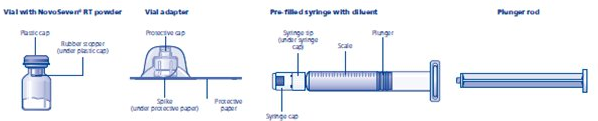
- Always use aseptic technique.
- Bring coagulation factor VIIa (white, lyophilized powder) and the specified volume of histidine (diluent) to room temperature, but not above 98.6° F (37° C). The specified volume of diluent corresponding to the amount of coagulation factor VIIa is as follows:
- 1 mg (1000 micrograms) vial + 1 mL Histidine diluent in pre-filled syringe
- 2 mg (2000 micrograms) vial + 2 mL Histidine diluent in pre-filled syringe
- 5 mg (5000 micrograms) vial + 5 mL Histidine diluent in pre-filled syringe
- 8 mg (8000 micrograms) vial + 8 mL Histidine diluent in pre-filled syringe
- Remove cap from the coagulation factor VIIa vial. Cleanse the rubber stopper with an alcohol swab and allow to dry prior to use.
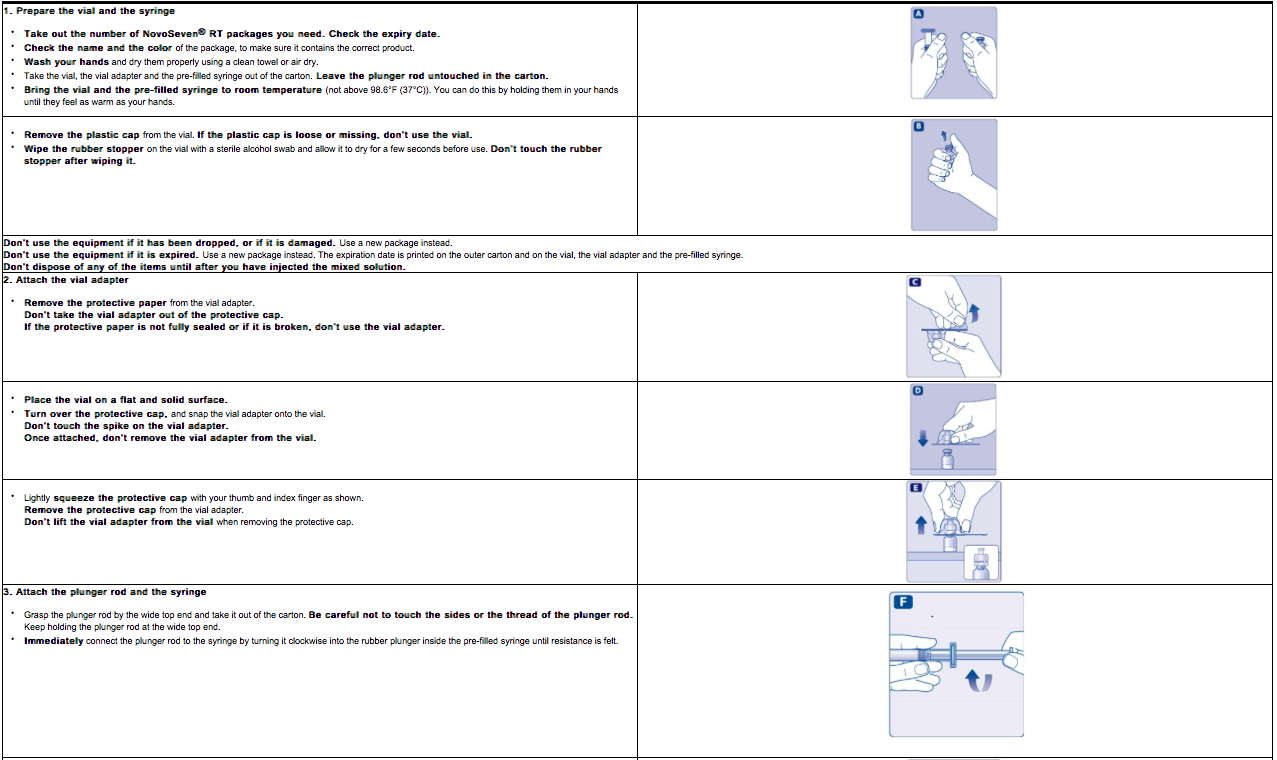
- Peel back the protective paper from the vial adapter. Do not remove the vial adapter from the package.
- Place the coagulation factor VIIa vial on a flat surface. While holding the vial adapter package, place the vial adapter over the coagulation factor VIIa vial and press down firmly on the package until the vial adapter spike penetrates the rubber stopper.
- Attach the plunger rod to the syringe. Turn the plunger rod clockwise into the plunger inside the pre-filled diluent syringe until resistance is felt. Remove the syringe cap from the pre-filled diluent syringe and screw onto the vial adapter.
- Push the plunger rod to slowly inject all the diluent into the vial. Keep the plunger rod pressed down and swirl the vial gently until the powder is dissolved. The reconstituted solution is a clear, colorless solution which may be stored fully assembled either at room temperature or refrigerated for up to 3 hours after reconstitution. After reconstitution with the specified volume of diluent, each vial contains approximately 1 mg per mL coagulation factor VIIa (1000 micrograms per mL).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Coagulation factor VIIa in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Coagulation factor VIIa in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Coagulation factor VIIa FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Coagulation factor VIIa in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Coagulation factor VIIa in pediatric patients.
Contraindications
None
Warnings
|
WARNING: SERIOUS THROMBOTIC ADVERSE EVENTS ASSOCIATED WITH THE USE OF NovoSeven RT OUTSIDE LABELED INDICATIONS
See full prescribing information for complete Boxed Warning.
* Arterial and venous thrombotic and thromboembolic events following administration of NovoSeven have been reported during postmarketing surveillance.
|
Thrombotic Events within the Licensed Indications
- Clinical trials within the approved indications revealed that thrombotic events of possible or probable relationship to NovoSeven occurred in 0.28% of bleeding episodes treated, with the incidence within hemophilia patients with inhibitors to be 0.20%, and in acquired hemophilia an incidence of 4%. Thrombotic events have been identified through postmarketing surveillance following coagulation factor VIIa use for each of the approved indications.2 The incidence of thrombotic events can not be determined from postmarketing data. Patients with disseminated intravascular coagulation (DIC), advanced atherosclerotic disease, crush injury, septicemia, or concomitant treatment with aPCCs/PCCs (activated or nonactivated prothrombin complex concentrates) have an increased risk of developing thrombotic events due to circulating tissue factor (TF) or predisposing coagulopathy. Caution should be exercised when administering coagulation factor VIIa to patients with an increased risk of thromboembolic complications. These include, but are not limited to, patients with a history of coronary heart disease, liver disease, disseminated intravascular coagulation, post-operative immobilization, elderly patients and neonates. In each of these situations, the potential benefit of treatment with coagulation factor VIIa should be weighed against the risk of these complications.
- Patients who receive coagulation factor VIIa should be monitored for development of signs or symptoms of activation of the coagulation system or thrombosis. When there is laboratory confirmation of intravascular coagulation or presence of clinical thrombosis, the coagulation factor VIIa dosage should be reduced or the treatment stopped, depending on the patient's symptoms.
Thrombotic Events outside the Licensed Indications
- NovoSeven has been studied in placebo controlled trials outside the approved indications to control bleeding in intracerebral hemorrhage, advanced liver disease, trauma, cardiac surgery, spinal surgery, and other therapeutic areas.
- Safety and effectiveness has not been established in these settings and the use is not approved by FDA. Two meta analyses of these pooled data indicate an increased risk of thrombotic events (10.0% in patients treated with NovoSeven versus 7.5% in placebo-treated patients). Arterial thromboembolic adverse events including myocardial infarction, myocardial ischemia, cerebral infarction and cerebral ischemia were statistically significantly increased with the use of NovoSeven compared to placebo (5.3 to 5.6% in subjects treated with NovoSeven versus 2.8 to 3.0% in placebo-treated patients). Other arterial thromboembolic events (such as retinal artery embolism, renal artery thrombosis, arterial thrombosis of limb, intracardiac thrombus, bowel infarction and intestinal infarction) have also been reported.3,4,5,6,7 While venous thromboembolic events such as deep venous thrombosis, portal vein thrombosis and pulmonary embolism have been reported in clinical trials, the meta analysis of these pooled data from placebo-controlled trials performed outside the currently approved indications did not suggest an increased risk of venous thromboembolic events in patients treated with NovoSeven versus placebo (4.8% in patients treated with NovoSeven versus 4.7% in placebo-treated patients).
- In spontaneous reports of women without a prior diagnosis of bleeding disorders receiving NovoSeven for uncontrolled post-partum hemorrhage, thrombotic events were observed. During this period, patients are at increased risk for thrombotic complications.
Post-Hemostatic Dosing
- Precautions should be exercised when coagulation factor VIIa is used for prolonged dosing.
Antibody Formation in Factor VII Deficient Patients
- Factor VII deficient patients should be monitored for prothrombin time (PT) and factor VII coagulant activity before and after administration of coagulation factor VIIa. If the factor VIIa activity fails to reach the expected level, or prothrombin time is not corrected, or bleeding is not controlled after treatment with the recommended doses, antibody formation may be suspected and analysis for antibodies should be performed.
Hypersensitivity Reactions
- Coagulation factor VIIa should be administered with caution in patients with known hypersensitivity to coagulation factor VIIa or any of its components, or in patients with known hypersensitivity to mouse, hamster, or bovine proteins.
Laboratory Tests
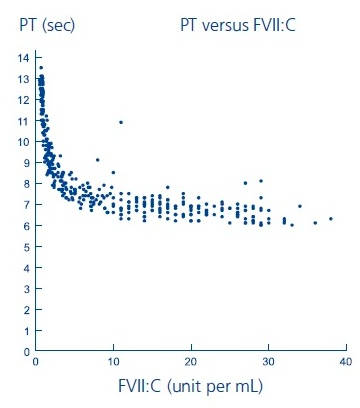
- Laboratory coagulation parameters (PT/INR, aPTT, FVII:C) have shown no direct correlation to achieving hemostasis. Assays of prothrombin time (PT/INR), activated partial thromboplastin time (aPTT), and plasma FVII clotting activity (FVII:C), may give different results with different reagents. Treatment with NovoSeven has been shown to produce the following characteristics:
- PT: As shown below, in patients with hemophilia A/B with inhibitors, the PT shortened to about a 7-second plateau at a FVII:C level of approximately 5 units per mL. For FVII:C levels > 5 units per mL, there is no further change in PT. The clinical relevance of prothrombin time shortening following coagulation factor VIIa administration is unknown.
- INR: NovoSeven has demonstrated the ability to normalize INR. However, INR values have not been shown to directly predict bleeding outcomes, nor has it been possible to demonstrate the impact of NovoSeven on bleeding times/volume in models of clinically-induced bleeding in healthy volunteers who had received Warfarin, when laboratory parameters (PT/INR, aPTT, thromboelastogram) have normalized.
- aPTT:While administration of NovoSeven shortens the prolonged aPTT in hemophilia A/B patients with inhibitors, normalization has usually not been observed in doses shown to induce clinical improvement. Data indicate that clinical improvement was associated with a shortening of aPTT of 15 to 20 seconds.
- FVIIa:C: FVIIa:C levels were measured two hours after NovoSeven administration of 35 micrograms per kg body weight and 90 micrograms per kg body weight following two days of dosing at two hour intervals. Average steady state levels were 11 and 28 units per mL for the two dose levels, respectively.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug product cannot be directly compared to rates in clinical trials of another drug, and may not reflect rates observed in practice.
- Thrombotic events following the administration of NovoSeven occurred in 0.3% of bleeding episodes treated, with the incidence in acquired hemophilia of 4% and in hemophilia patients of 0.2% in clinical trials within the approved indications.
- Adverse reactions observed in clinical trials for all labeled indications of NovoSeven included pyrexia, injection site reaction, headache, hypertension, nausea, vomiting, pain, edema, rash (including allergic dermatitis and rash erythematous), pruritus, urticaria, hypersensitivity, cerebral artery occlusion, cerebrovascular accident, pulmonary embolism, deep vein thrombosis, angina pectoris, increased levels of fibrin degradation products, disseminated intravascular coagulation and related laboratory findings including elevated levels of D-dimer and AT-III, thrombosis at i.v. site, non-specified thrombosis, thrombophlebitis, superficial thrombophlebitis.
- The following sections describe the adverse event profile observed during clinical studies for each of the labeled indications.
Hemophilia A or B Patients with Inhibitors
- Two studies (Studies 1 and 2) are described for hemophilia A or B patients with inhibitors treated for bleeding episodes. The table below lists adverse reactions that were reported in ≥2% of the 298 patients with hemophilia A or B with inhibitors that were treated with NovoSeven for 1,939 bleeding episodes.
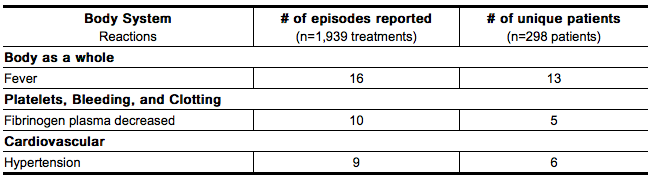
- Other reactions reported in 1% of patients were: allergic reaction, coagulation disorder, DIC, edema, fibrinolysis increased, headache, injection site reaction, pain, pruritus, purpura, rash, thrombosis and therapeutic response decreased.
- Serious adverse reactions occurred in approximately 3% of the patients and included thrombosis, pain, thrombophlebitis, pulmonary embolism, therapeutic response decreased, cerebrovascular disorder, angina pectoris, DIC, anaphylactic shock and hepatic function abnormal. The serious adverse reactions of DIC and therapeutic response decreased had a fatal outcome.
Surgery Studies
- Two clinical trials (Studies 3 and 4) were conducted to evaluate the safety and efficacy of NovoSeven administration during and after surgery in hemophilia A or B patients with inhibitors.
- In one study (Study 3), two patients had adverse reactions (acute post-operative hemarthrosis and internal jugular thrombosis). No deaths occurred during the study.
- In another study (Study 4), four of 24 patients had serious adverse reactions, all being decreased therapeutic response. Two reactions were observed in each treatment arm (bolus injection and continuous infusion). No deaths occurred during the study period.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of NovoSeven. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship.
- These adverse reactions were reported following the use of NovoSeven in labeled and unlabeled indications that included individuals with and without coagulopathy: high D-dimer levels and coagulopathy, thrombosis, thrombophlebitis, arterial thrombosis, and thromboembolic events including myocardial ischemia, myocardial infarction, cerebral ischemia, cerebral infarction, renal artery thrombosis, intracardiac thrombus, portal vein thrombosis, thrombophlebitis, peripheral ischemia, deep vein thrombosis and related pulmonary embolism, injection site pain, headache, nausea and isolated cases of hypersensitivity/allergic reactions including anaphylactic shock, flushing, urticaria, rash, and angioedema
- Fatal and non-fatal thromboembolic events have been reported with use of NovoSeven when used for off-label or labeled indications.
- There have been no confirmed reports on inhibitory antibodies against NovoSeven or FVII in patients with congenital hemophilia A or B with alloantibodies.
- The Hemophilia and Thrombosis Research Society (HTRS) Registry surveillance program is designed to collect data on the treatment of congenital and acquired bleeding disorders.8 All prescribers can obtain information regarding contribution of patient data to this program by calling 1-877-362-7355 or at www.novosevensurveillance.com.
Congenital Factor VII Deficiency
- Data collected from the compassionate/emergency use programs, the published literature, a pharmacokinetics study, and the Hemophilia and Thrombosis Research Society (HTRS) registry showed that at least 75 patients with Factor VII deficiency had received NovoSeven - 70 patients for 124 bleeding episodes, surgeries, or prophylaxis regimens; 5 patients in the pharmacokinetics trial.
- In the compassionate/emergency use programs, 1 non-serious adverse reaction (intracranial hypertension) and 1 serious adverse reaction (IgG antibody against rFVIIa and FVII) were reported. One adverse reaction (localized phlebitis) was reported in the literature. No adverse reactions were reported in the pharmacokinetics reports or for the HTRS registry. No thromboembolic complications were reported for the 75 patients included here.
- As with all therapeutic proteins, there is a potential for immunogenicity. In compassionate/emergency use programs patients with factor VII deficiency formation of antibodies against NovoSeven and FVII (frequency: common (≥ 1/100 to < 1/10)) have been reported. In some cases, the antibodies showed inhibitory effect in vitro. Risk factors that may have contributed to antibody development including previous treatment with human plasma and/or plasma-derived factor VII, severe mutation of FVII gene, and overdose of NovoSeven, were present. Patients with factor VII deficiency treated with NovoSeven should be monitored for factor VII antibodies.
Acquired Hemophilia
- Data collected from four compassionate use programs, the HTRS registry, and the published literature showed that 139 patients with acquired hemophilia received NovoSeven for 204 bleeding episodes, surgeries and traumatic injuries.
- Of these 139 patients, 6 experienced 8 serious adverse reactions. Thrombotic serious adverse events included cerebral artery occlusion, cerebral ischemia, angina pectoris, myocardial infarction, pulmonary embolism and deep vein thrombosis.
- Additional serious adverse reactions included shock and cerebrovascular accident. Three of the serious adverse reactions had a fatal outcome (shock, cerebral artery occlusion and myocardial infarction).
Drug Interactions
Coagulation Factor Concentrates
- The risk of a potential interaction between coagulation factor VIIa and coagulation factor concentrates has not been adequately evaluated in preclinical or clinical studies. Simultaneous use of activated prothrombin complex concentrates or prothrombin complex concentrates should be avoided.
Infusion Solutions
- Coagulation factor VIIa should not be mixed with infusion solutions.
Coagulation Factor XIII A-Subunit (Recombinant) (rFXIII)
- Thrombosis may occur if coagulation factor VIIa is administered concomitantly with Coagulation Factor XIII A-Subunit (Recombinant).
Use in Specific Populations
Pregnancy
- Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. Coagulation factor VIIa should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Treatment of rats and rabbits with NovoSeven in reproduction studies has been associated with mortality at doses up to 6 mg per kg body weight and 5 mg per kg body weight. At 6 mg per kg body weight in rats, the abortion rate was 0 out of 25 litters; in rabbits at 5 mg per kg body weight, the abortion rate was 2 out of 25 litters. Twenty-three out of 25 female rats given 6 mg per kg body weight of NovoSeven gave birth successfully, however, two of the 23 litters died during the early period of lactation. No evidence of teratogenicity was observed after dosing with NovoSeven.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Coagulation factor VIIa in women who are pregnant.
Labor and Delivery
- NovoSeven was administered to a FVII deficient patient (25 years of age, 66 kg) during a vaginal delivery (36 micrograms per kg body weight) and during a tubal ligation (90 micrograms per kg body weight). No adverse reactions were reported during labor, vaginal delivery, or the tubal ligation.
- There are no adequate and well-controlled studies in labor, delivery, and postpartum periods. In spontaneous reports of women without a prior diagnosis of bleeding disorders receiving NovoSeven for uncontrolled post-partum hemorrhage, thrombotic events were observed. During this period, patients are at increased risk for thrombotic complications. It is not known to what extent NovoSeven contributed to the occurrence of these events.
Nursing Mothers
- It is not known whether coagulation factor VIIa is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Clinical trials enrolling pediatric patients were conducted with dosing determined according to body weight and not according to age. The safety and effectiveness of coagulation factor VIIa has not been studied to determine if there are differences among various age groups, from infants to adolescents (0 to 16 years of age).
Geriatic Use
Clinical studies of NovoSeven in congenital factor deficiencies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Coagulation factor VIIa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Coagulation factor VIIa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Coagulation factor VIIa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Coagulation factor VIIa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Coagulation factor VIIa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Coagulation factor VIIa in patients who are immunocompromised.
Administration and Monitoring
Administration
- For intravenous bolus administration only.
Monitoring
There is limited information regarding Coagulation factor VIIa Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Coagulation factor VIIa and IV administrations.
Overdosage
- There are no adequate and well controlled studies to support the safety or efficacy of using higher than labeled doses in the indicated populations.
Dose limiting toxicities of coagulation factor VIIa have not been investigated in clinical trials. The following are examples of accidental overdose.
Congenital Factor VII Deficiency
- A newborn female with congenital factor VII deficiency was administered an overdose of NovoSeven (single dose: 800 micrograms per kg body weight). Following additional administration of NovoSeven and various plasma products, antibodies against rFVIIa were detected, but no thrombotic complications were reported. A Factor VII deficient male (83 years of age, 111.1 kg) received two doses of 324 micrograms per kg body weight (10-20 times the recommended dose) and experienced a thrombotic event (occipital stroke).
Hemophilia A or B with Inhibitors
- One hemophilia B patient (16 years of age, 68 kg) received a single dose of 352 micrograms per kg body weight and one hemophilia A patient (2 years of age, 14.6 kg) received doses ranging from 246 micrograms per kg body weight to 986 micrograms per kg body weight on five consecutive days. There were no reported complications in either case.
Pharmacology
There is limited information regarding Coagulation factor VIIa Pharmacology in the drug label.
Mechanism of Action
- Coagulation factor VIIa is recombinant Factor VIIa and, when complexed with tissue factor can activate coagulation Factor X to Factor Xa, as well as coagulation Factor IX to Factor IXa. Factor Xa, in complex with other factors, then converts prothrombin to thrombin, which leads to the formation of a hemostatic plug by converting fibrinogen to fibrin and thereby inducing local hemostasis. This process may also occur on the surface of activated platelets.
Structure
There is limited information regarding Coagulation factor VIIa Structure in the drug label.
Pharmacodynamics
- The effect of coagulation factor VIIa upon coagulation in patients with or without hemophilia has been assessed in different model systems. In an in vitro model of tissue-factor-initiated blood coagulation (Figure A),10 the addition of rFVIIa increased both the rate and level of thrombin generation in normal and hemophilia A blood, with an effect shown at rFVIIa concentrations as low as 10 nM. In this model, fresh human blood was treated with corn trypsin inhibitor (CTI) to block the contact pathway of blood coagulation. Tissue factor (TF) was added to initiate clotting in the presence and absence of rFVIIa for both types of blood.
- In a separate model, and in line with previous reports,11 escalating doses of rFVIIa in hemophilia plasma demonstrate a dose-dependent increase in thrombin generation (Figure B). In this model, platelet rich normal and hemophilia plasma was adjusted with autologous plasma to 200,000 platelets/microliter. Coagulation was initiated by addition of tissue factor and CaCl2. Thrombin generation was measured in the presence of a thrombin substrate and various added concentrations of rFVIIa.
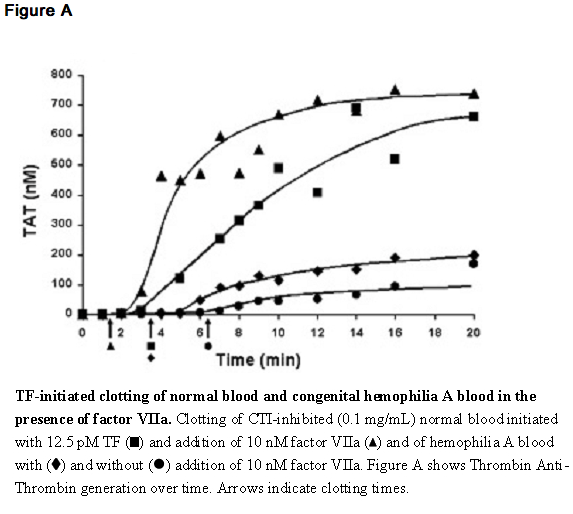
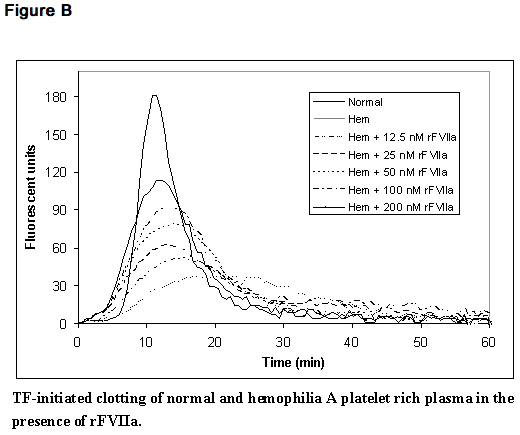
Pharmacokinetics
Healthy Subjects
- The pharmacokinetics of NovoSeven was investigated in 35 healthy Caucasian and Japanese subjects in a dose-escalation study. Subjects were stratified according to gender and ethnic group and dosed with 40, 80 and 160 micrograms per kg NovoSeven.12 * The pharmacokinetics of rFVII were linear over the dose range of 40 to 180 micrograms per kg. Pharmacokinetics were similar across gender and ethnic groups.
- Mean steady state volume of distribution ranged from 130 to 165 mL per kg, mean values of clearance ranged from 33 to 37 mL per hr x kg, and mean terminal half-life ranged from 3.9 to 6.0 hours.
Hemophilia A or B
- Single‑dose pharmacokinetics of NovoSeven (17.5, 35, and 70 micrograms per kg) exhibited dose-proportional behavior in 15 subjects with hemophilia A or B.13 Factor VII clotting activities were measured in plasma drawn prior to and during a 24‑hour period after NovoSeven administration. The median apparent volume of distribution at steady state was 103 mL per kg (range 78-139). Median clearance was 33 mL per kg per hr (range 27-49). The median residence time was 3.0 hours (range 2.4-3.3), and the t1/2 was 2.3 hours (range 1.7-2.7). The median in vivo plasma recovery was 44% (30-71%). The products coagulation factor VIIa and NovoSeven are pharmacokinetically equivalent.14
- In a bolus single-dose pharmacokinetic study, 5 male adults (90 micrograms per kg) and 10 male pediatric (2-12 years) patients (crossover, 90 and 180 micrograms per kg) with severe hemophilia A (10 of 18 subjects had inhibitors) received NovoSeven.15 The PK of rFVII following 90 and 180 micrograms per kg IV dose in children indicated dose linearity. Based on the FVII:C assay, the terminal half-life of NovoSeven was 2.6 hrs in pediatric patients and 3.1 hrs in adults. Based on the 90 microgram/kg dose, the total clearance of NovoSeven in adults and children was 2767 ± 385 mL per hr (37.6 ± 13.1 mL per hr per kg) and 1375 ± 396 mL per hr (57.3 ± 9.5 mL per hr per kg), respectively. The volume of distribution at steady state (Vss) in adults and children was 121 ± 30 and 153 ± 29 mL per kg, respectively.
Congenital Factor VII deficiency
- Single dose pharmacokinetics of NovoSeven in congenital Factor VII deficiency, at doses of 15 and 30 micrograms per kg body weight, showed no significant difference between the two doses used with regard to dose-independent parameters: total body clearance (70.8-79.1 mL per hr x kg), volume of distribution at steady state (280-290 mL per kg), mean residence time (3.75-3.80 hr), and half-life (2.82-3.11 hr). The mean in vivo plasma recovery was approximately 20% (18.9%-22.2%).
- The normal Factor VII plasma concentration is 0.5 micrograms per mL. Factor VII levels of 15-25% (0.075 – 0.125 micrograms per mL) are generally sufficient to achieve normal hemostasis.16 For example, a 70 kg individual with FVII deficiency (plasma volume of approximately 3000 mL) would thus require 3.2 - 5.4 micrograms per kg of coagulation factor VIIa to secure hemostasis, assuming 100% recovery but, since the mean plasma recovery for NovoSeven is 20% for FVII-deficient patients, a coagulation factor VIIa dose range of 16-27 micrograms per kg would be required to achieve sufficient FVII plasma levels for hemostasis, which is consistent with the recommended dose range.
- No adverse reactions were reported in the pharmacokinetics for congenital factor VII deficiency.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Two mutagenicity studies have given no indication of carcinogenic potential for NovoSeven. The clastogenic activity of NovoSeven was evaluated in both in vitro studies (i.e., cultured human lymphocytes) and in vivo studies (i.e., mouse micronucleus test). Neither of these studies indicated clastogenic activity of NovoSeven. Other gene mutation studies have not been performed with NovoSeven RT (e.g., Ames test). No chronic carcinogenicity studies have been performed with coagulation factor VIIa.
- A reproductive study in male and female rats at dose levels up to 3.0 mg per kg per day had no effect on mating performance, fertility, or litter characteristics.
- Treatment of rats and rabbits with NovoSeven in reproduction studies has been associated with mortality at doses up to 6 mg per kg and 5 mg per kg. At 6 mg per kg in rats, the abortion rate was 0 out of 25 litters; in rabbits at 5 mg per kg, the abortion rate was 2 out of 25 litters. Twenty-three out of 25 female rats given 6 mg per kg of NovoSeven gave birth successfully, however, two of the 23 litters died during the early period of lactation. No evidence of teratogenicity was observed after dosing with NovoSeven.
Animal Toxicology and/or Pharmacology
- In a monkey cardiovascular safety pharmacology model evaluating the combination of excessive doses of Coagulation Factor XIII A-Subunit (Recombinant) (585 IU/kg, 17 times the expected human dose) in combination with rFVIIa (1000 mcg/kg, 11 times the expected human dose), one of the twelve monkeys died 4 hours after treatment due to thrombosis. Procoagulant risk factors, including 6 indwelling catheters per monkey and the induction of anesthesia, may have complicated the study results. It is unclear whether the mortality was related to the overdose of one or both products, or a specific interaction between them. Nonclinical and clinical studies with the combination of rFXIII and coagulation factor VIIa at recommended human doses have not been performed.
Clinical Studies
- No direct comparisons to other coagulation products have been conducted, therefore no conclusions regarding the comparative safety or efficacy can be made.
Hemophilia A or B with Inhibitors
Open Protocol Use
- The largest number of patients who received NovoSeven during the investigational phase of product development were in an open protocol study (Study 1)17,18,19 that began enrollment in 1988, shortly after the completion of the pharmacokinetic study. * These patients included persons with hemophilia types A or B (with or without inhibitors), persons with acquired inhibitors to Factor VIII or Factor IX, and a few FVII deficient patients. The clinical situations were diverse and included muscle/joint bleeds, mucocutaneous bleeds, surgical prophylaxis, intracerebral bleeds, and other emergent situations. Dose schedules were suggested by Novo Nordisk, but they were subject to the option of the investigator. Clinical outcomes were not reported in a standardized manner. Therefore, the clinical data from Study 1 are problematic for the evaluation of the safety and efficacy of the product by statistical methods.
Dosing Study
- The dosing study (Study 2)20 was a double-blind, randomized comparison trial of two dose levels of NovoSeven in the treatment of joint, muscle and mucocutaneous hemorrhages in hemophilia A and B patients with and without inhibitors. Patients received NovoSeven as soon as they could be evaluated in the treatment centers (4 to 18 hours after experiencing a bleed). Thirty-five patients were treated at the 35 micrograms per kg dose (59 joint, 15 muscle and 5 mucocutaneous bleeding episodes) and 43 patients were treated at the 70 micrograms per kg dose (85 joint and 14 muscle bleeding episodes).
- Dosing was to be repeated at 2.5 hour intervals but ranged up to four hours for some patients. Efficacy was assessed at 12 ± 2 hours or at end of treatment, whichever occurred first. Based on a subjective evaluation by the investigator, the respective efficacy rates for the 35 and 70 micrograms per kg groups were: excellent 59% and 60%, effective 12% and 11%, and partially effective 17% and 20%. The average number of injections required to achieve hemostasis was 2.8 and 3.2 for the 35 and 70 micrograms per kg groups, respectively.
- One patient in the 35 micrograms per kg group and three in the 70 micrograms per kg group experienced serious adverse events that were not considered related to NovoSeven. Two unrelated deaths occurred; one patient died of AIDS and the other of intracranial hemorrhage secondary to trauma.
Surgery Studies
- Two clinical trials (Studies 3 and 4) were conducted to evaluate the safety and efficacy of NovoSeven administration during and after surgery in hemophilia A or B patients with inhibitors.
- One study (Study 3) was a randomized, double-blind, parallel group clinical trial (29 patients with hemophilia A or B and inhibitors or acquired inhibitors to FVIII/FIX, undergoing major or minor surgical procedures).21 Patients received bolus intravenous NovoSeven (either 35 micrograms per kg, N=15; or 90 micrograms per kg, N=14) prior to surgery, intra-operatively as required, then every 2 hours for the following 48 hours beginning at closure of the wound. Additional doses were administered every 2 to 6 hours up to an additional 3 days to maintain hemostasis.
- After a maximum of 5 days of double-blind treatment, therapy could be continued in an open-label manner if necessary (90 micrograms per kg NovoSeven every 2-6 hours). Efficacy was assessed during the intra-operative period, and post-operatively from the time of wound closure (Hour 0) through Day 5.
- When efficacy assessments at each time point were tabulated by a last value carried forward approach (patients who completed the study early having achieved effective hemostasis were counted as “effective” and those who discontinued due to treatment failure or adverse events were counted as “ineffective” at each time point thereafter), the results at the end of the 5-day double-blind treatment period were as summarized in the table below. Twenty-three patients successfully completed the entire study (including the open-label period after the 5-day double blind period) with satisfactory hemostasis.
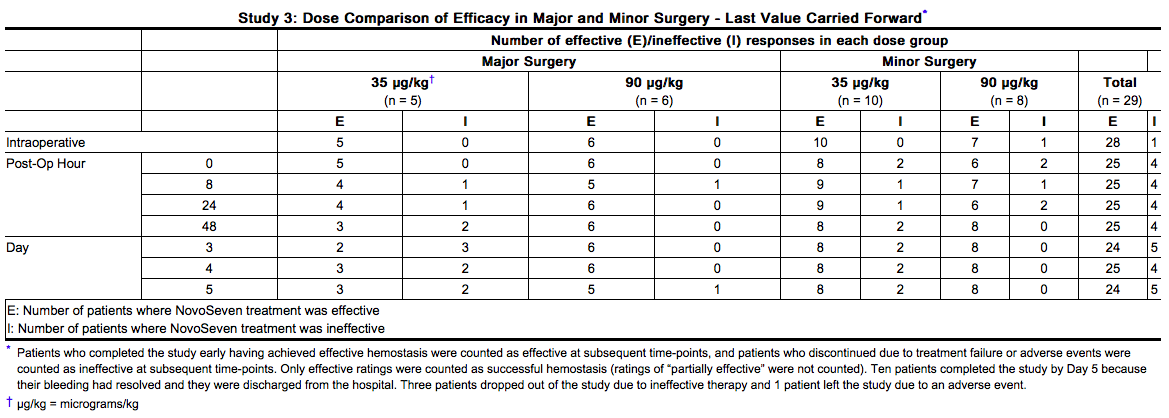
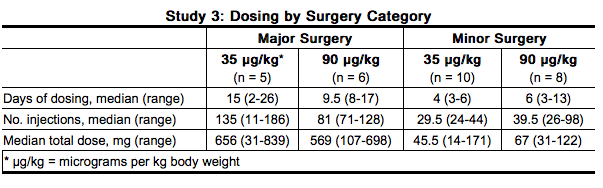
- Study 4 was an open-label, randomized, parallel trial conducted to compare the safety and efficacy of bolus intravenous injection (N=12) and continuous intravenous infusion (N=12) administration of NovoSeven in hemophilia A or B patients with inhibitors who were undergoing elective major surgery. The types of surgeries that were performed included knee (N=13), hip (N=3), abdomen/lower pelvis (N=2), groin/inguinal area (N=2), circumcision (N=1), eye (N=1), frontal/temporal region of cranium (N=1), and oral cavity (N=1).
- Prior to surgery, a 90 micrograms per kg bolus dose of NovoSeven was administered to both bolus and continuous infusion groups. The bolus injection group then received 90 micrograms per kg NovoSeven by bolus intravenous injection every 2 hours during the procedure and for the first 5 days, then every 4 hours from Day 6 to Day 10. The continuous infusion group received 50 micrograms per kg per hr NovoSeven by continuous intravenous infusion for the first 5 days, and infusion of 25 micrograms per kg per hr from Day 6 to Day 10. For both NovoSeven-treated groups, two bolus rescue doses of 90 micrograms per kg were permitted during any 24-hour period.
- The bolus injection (90 micrograms/kg) and continuous infusion (50 micrograms/kg/h) treatment groups showed comparable efficacy in achieving and maintaining hemostasis in major surgery from wound closure through Day 10. For the Global Hemostasis Treatment Evaluation for overall success in achieving and maintaining hemostasis at the end of the study period, treatment was rated as being effective in 9 patients (75%) and ineffective in 3 patients (25%) for both treatment groups.
- When efficacy assessments at each time point were tabulated by a last value carried forward approach (patients who completed the study early having achieved effective hemostasis were counted as “effective” at each time point, and those who discontinued due to treatment failure counted as “ineffective” at each time point thereafter), the results were as summarized in the table below.
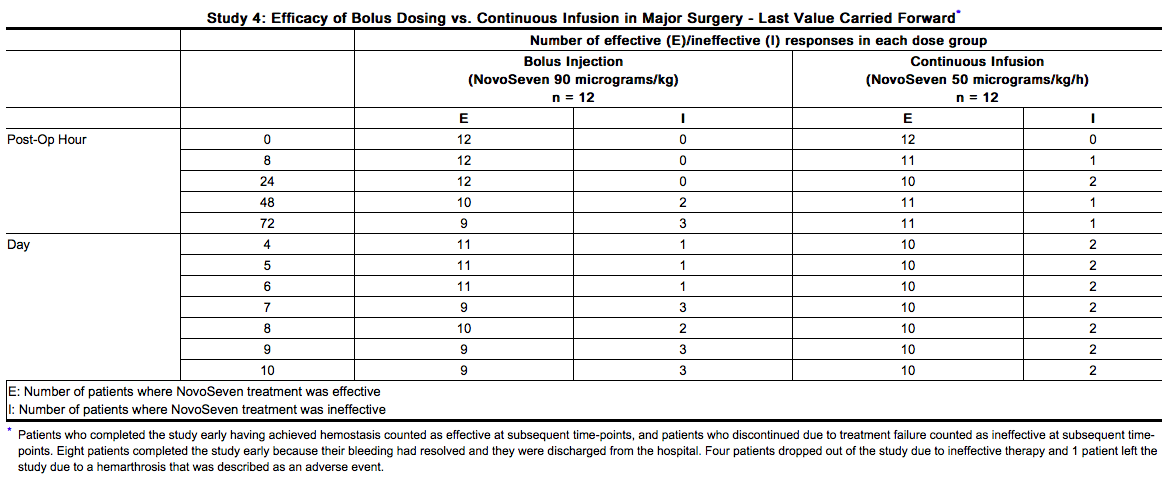
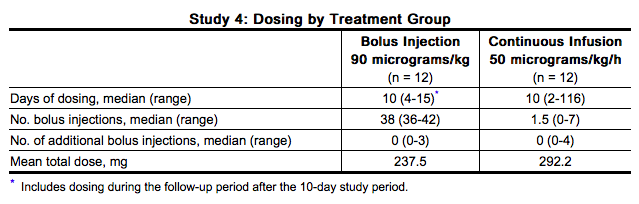
Congenital Factor VII Deficiency
- Data were collected from the published literature and internal sources for 70 patients with Factor VII deficiency treated with NovoSeven for 124 bleeding episodes, surgeries, or prophylaxis regimens. Thirty-two of these patients were enrolled in emergency and compassionate use trials conducted by Novo Nordisk (43 non-surgical bleeding episodes, 26 surgeries); 35 were reported in the published literature (20 surgeries, 10 non-surgical bleeding episodes, 4 cases of caesarean section or vaginal birth, and 10 cases of long-term prophylaxis, and 1 case of on-demand therapy); and 3 were from a registry maintained by the Hemophilia and Thrombosis Research Society (9 bleeding episodes, 1 surgery). Dosing ranged from 6 to 98 micrograms per kg administered every 2-12 hours (except for prophylaxis, where doses were administered from 2 times per day up to 2 times per week). Patients were treated with an average of 1-10 doses. Treatment was effective (bleeding stopped or treatment was rated as effective by the physician) in 93% of episodes (90% for trial patients, 98% for published patients, 90% for HTRS registry patients).
Acquired Hemophilia
- Data were collected from four studies in the compassionate use program conducted by Novo Nordisk and the Hemophilia and Thrombosis Research Society (HTRS) registry. A total of 70 patients with acquired hemophilia were treated with NovoSeven for 113 bleeding episodes, surgeries, or traumatic injuries. Sixty-one of these patients were from the compassionate use program with 100 bleeding episodes (68 non-surgical and 32 surgical bleeding episodes) and 9 patients were from the HTRS registry with 13 bleeding episodes (8 non-surgical, 3 surgical and 2 episodes classified as other). Concomitant use of other hemostatic agents occurred in 29/70 (41%); 13 (19%) received more than one hemostatic agent. The most common hemostatic agents used were antifibrinolytics, Factor VIII and activated prothrombin complex concentrates.
- The compassionate use programs and the HTRS registry were not designed to select doses or compare first-line efficacy or efficacy when used after failure of other hemostatic agents (salvage treatment). A dose response was not seen in doses ranging from 70-90 micrograms per kg.
- The mean dose of NovoSeven administered was 90 micrograms per kg (range: 31 to 197 micrograms per kg); the mean number of injections per day was 6 (range: 1 to 10 injections per day). Overall efficacy i.e., effective and partially effective outcomes, was 87/112 (78%); with 77/100 (77%) efficacy in the compassionate use programs and 10/12 (83%) efficacy in the HTRS registry. In the compassionate use programs, overall efficacy for the first-line treatment was 38/44 (86%) compared to 39/56 (70%) when used as salvage treatment.
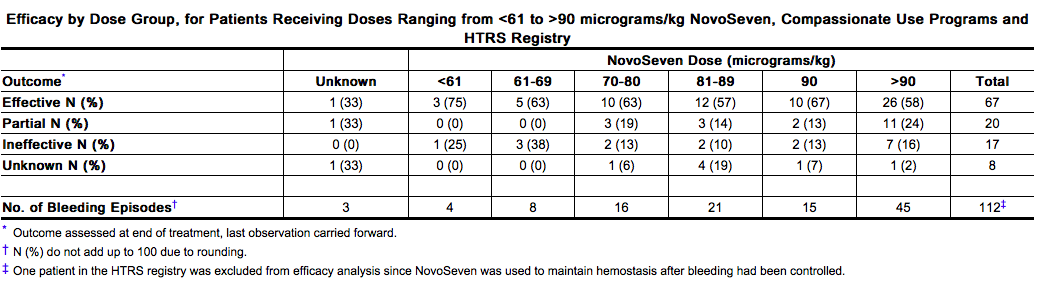
How Supplied
- Coagulation Factor VIIa (Recombinant), is supplied as a room temperature stable, white, lyophilized powder in single‑use vials, one vial per carton. The diluent for reconstitution of coagulation factor VIIa is a 10 mmol solution of L-histidine in water for injection and is supplied as a clear colorless solution, and referred to as the histidine diluent. The histidine diluent is provided in either a vial or pre-filled diluent syringe.
- The amount of rFVIIa in milligrams and in micrograms is stated on the label.
coagulation factor VIIa package containing 1 vial of coagulation factor VIIa powder and 1 vial of histidine diluent:
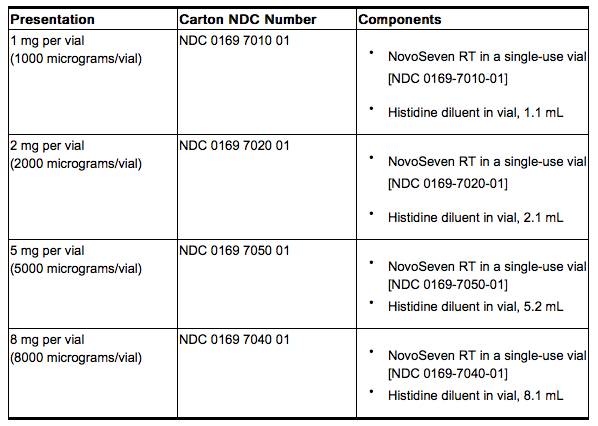
- Coagulation factor VIIa with MixPro® package containing 1 vial of coagulation factor VIIa powder and 1 pre-filled histidine diluent syringe with sterile vial adapter which serves as an alternative needleless reconstitution system:
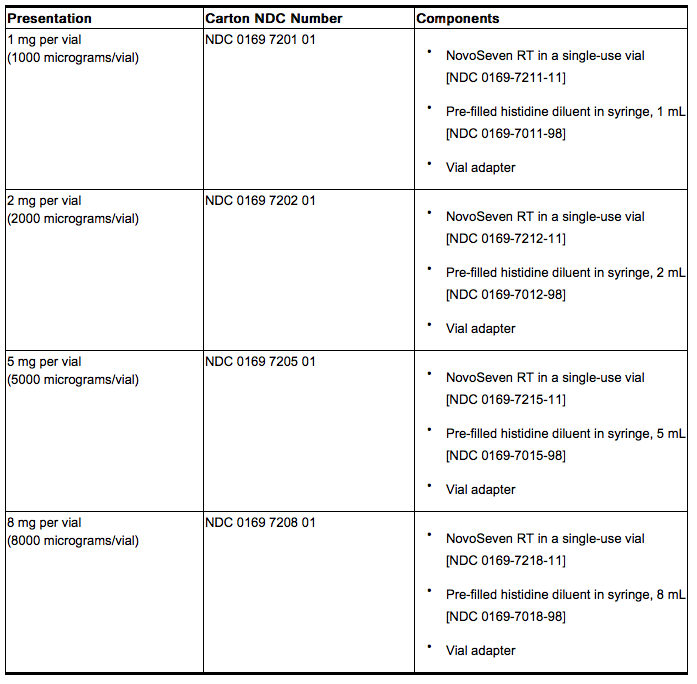
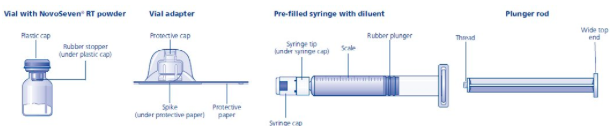
- The coagulation factor VIIa and histidine diluent vials are made of glass, closed with a latex-free, chlorobutyl rubber stopper, and covered with an aluminum cap. The pre-filled diluent syringes are made of glass, with a latex-free siliconised bromobutyl rubber plunger. The closed vials and pre-filled diluent syringes are equipped with a tamper-evident snap-off cap which is made of polypropylene. A vial adapter with 25 micrometer filter is provided with the prefilled diluent syringe.
Storage
- Prior to reconstitution, store coagulation factor VIIa powder and histidine diluent between 2-25°C (36-77°F). Do not freeze. Store protected from light. Do not use past the expiration date.
- After reconstitution, store coagulation factor VIIa either at room temperature or refrigerated for up to 3 hours. Do not freeze reconstituted coagulation factor VIIa or store in syringes.
Images
Drug Images
{{#ask: Page Name::Coagulation factor VIIa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Coagulation factor VIIa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients receiving coagulation factor VIIa the benefits and risks associated with treatment.
- Advise patients about the early signs of hypersensitivity reactions, including hives, urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis.
- Advise patients about the signs of thrombosis, including new onset swelling and pain in the limbs or abdomen, new onset chest pain, shortness of breath, loss of sensation or motor power, or altered consciousness or speech.
- Advise patients to immediately seek medical help if any of the above signs or symptoms occur.


Precautions with Alcohol
Alcohol-Coagulation factor VIIa interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- NovoSeven RT[1]
Look-Alike Drug Names
Novoseven - Novoseven RT[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "NovoSeven RT".
- ↑ "https://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_01.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_02.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_03.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_04.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_05.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_06.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_07.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_08.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_09.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_10.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_11.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_12.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_13.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_14.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_15.jpg
}}
{{#subobject:
|Label Page=Coagulation factor VIIa |Label Name=Coagulation factor_label_16.jpg
}}