Clofarabine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 94: | Line 94: | ||
'''Enterocolitis''' | '''Enterocolitis''' | ||
* Fatal and serious cases of [[enterocolitis]], including [[neutropenic colitis]], [[cecitis]], and [[C. difficile colitis]], have occurred during treatment with clofarabine. This has occurred more frequently within 30 days of treatment, and in the setting of combination chemotherapy. Enterocolitis may lead to [[necrosis]], [[perforation]], [[hemorrhage]] or [[sepsis]] complications . Monitor patients for signs and symptoms of enterocolitis and treat promptly. | * Fatal and serious cases of [[enterocolitis]], including [[neutropenic colitis]], [[cecitis]], and [[Clostridium difficile infection|C. difficile colitis]], have occurred during treatment with clofarabine. This has occurred more frequently within 30 days of treatment, and in the setting of combination chemotherapy. Enterocolitis may lead to [[necrosis]], [[perforation]], [[hemorrhage]] or [[sepsis]] complications . Monitor patients for signs and symptoms of enterocolitis and treat promptly. | ||
'''Skin Reactions''' | '''Skin Reactions''' | ||
* Serious and fatal cases of [[Stevens-Johnson syndrome]] (SJS) and [[toxic epidermal necrolysis]] (TEN), have been reported. Discontinue Clofarabine for exfoliative or | * Serious and fatal cases of [[Stevens-Johnson syndrome]] (SJS) and [[toxic epidermal necrolysis]] (TEN), have been reported. Discontinue Clofarabine for [[rash|exfoliative]] or [[rash|bullous rash]], or if SJS or TEN is suspected. | ||
'''Embryo-fetal Toxicity''' | '''Embryo-fetal Toxicity''' | ||
| Line 120: | Line 120: | ||
* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | * Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
* The data described below reflect exposure to Clofarabine in 115 pediatric patients with relapsed or refractory Acute Lymphoblastic Leukemia (ALL) (70 patients) or [[Acute Myelogenous Leukemia]] (AML) (45 patients). | * The data described below reflect exposure to Clofarabine in 115 pediatric patients with relapsed or refractory [[Acute Lymphoblastic Leukemia]] (ALL) (70 patients) or [[Acute Myelogenous Leukemia]] (AML) (45 patients). | ||
* In total, 115 pediatric patients treated in clinical trials received the recommended dose of Clofarabine 52 mg/m2 daily × 5. The median number of cycles was 2. The median cumulative amount of Clofarabine received by pediatric patients during all cycles was 540 mg. | * In total, 115 pediatric patients treated in clinical trials received the recommended dose of Clofarabine 52 mg/m2 daily × 5. The median number of cycles was 2. The median cumulative amount of Clofarabine received by pediatric patients during all cycles was 540 mg. | ||
* The most common adverse reactions occurring in 10% or more of patients treated with Clofarabine are: [[nausea]], [[vomiting]], [[diarrhea]], [[febrile neutropenia]], [[headache]], [[rash]], [[pruritus]], pyrexia, [[fatigue]], palmar-plantar erythrodysesthesia syndrome, [[anxiety]], flushing, and mucosal inflammation. | * The most common adverse reactions occurring in 10% or more of patients treated with Clofarabine are: [[nausea]], [[vomiting]], [[diarrhea]], [[febrile neutropenia]], [[headache]], [[rash]], [[pruritus]], pyrexia, [[fatigue]], [[Palmar plantar erythrodysesthesia|palmar-plantar erythrodysesthesia syndrome]], [[anxiety]], flushing, and mucosal inflammation. | ||
* Table 1 lists adverse reactions by System Organ Class, including severe or life-threatening (NCI CTC Grade 3 or Grade 4), reported in ≥ 5% of the 115 patients in the 52 mg/m2/day dose group (pooled analysis of pediatric patients with ALL and AML). More detailed information and follow-up of certain events is given below. | * Table 1 lists adverse reactions by System Organ Class, including severe or life-threatening (NCI CTC Grade 3 or Grade 4), reported in ≥ 5% of the 115 patients in the 52 mg/m2/day dose group (pooled analysis of pediatric patients with ALL and AML). More detailed information and follow-up of certain events is given below. | ||
| Line 132: | Line 132: | ||
* The following less common adverse reactions have been reported in 1–4% of the 115 pediatric patients with ALL or AML: | * The following less common adverse reactions have been reported in 1–4% of the 115 pediatric patients with ALL or AML: | ||
:*''Gastrointestinal Disorders'': cecitis, [[pancreatitis]] | :*''Gastrointestinal Disorders'': [[cecitis]], [[pancreatitis]] | ||
:*''Hepatobiliary Disorders'': [[hyperbilirubinemia]] | :*''Hepatobiliary Disorders'': [[hyperbilirubinemia]] | ||
| Line 138: | Line 138: | ||
:*''Immune System Disorders'': [[hypersensitivity]] | :*''Immune System Disorders'': [[hypersensitivity]] | ||
:*''Infections and Infestations'': bacterial infection, Enterococcal bacteremia, Escherichia bacteremia, Escherichia sepsis, fungal infection, fungal sepsis, gastroenteritis adenovirus, infection, [[influenza]], parainfluenza virus infection, pneumonia | :*''Infections and Infestations'': bacterial infection, [[enterococcus|Enterococcal bacteremia]], [[escherichia|Escherichia bacteremia]], [[escherichia|Escherichia sepsis]], fungal infection, fungal sepsis, [[adenovirus|gastroenteritis adenovirus]], infection, [[influenza]], [[parainfluenza|parainfluenza virus infection]], fungal pneumonia, pneumonia primary atypical, {{RSV|Respiratory syncytial virus infection]], [[sinusitis]], [[staphylococcus|staphylococcal infection]] | ||
:*''Investigations'': blood creatinine increased | :*''Investigations'': [[creatinine|blood creatinine increased]] | ||
:*''Psychiatric Disorders'': mental status change | :*''Psychiatric Disorders'': mental status change | ||
| Line 151: | Line 151: | ||
|postmarketing=* The following adverse reactions have been identified during post-approval use of Clofarabine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) reported frequency of the reaction, or (3) strength of causal connection to Clofarabine. | |postmarketing=* The following adverse reactions have been identified during post-approval use of Clofarabine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) reported frequency of the reaction, or (3) strength of causal connection to Clofarabine. | ||
:*''Gastrointestinal disorders'': Gastrointestinal hemorrhage including fatalities. | :*''Gastrointestinal disorders'': [[Gastrointestinal hemorrhage]] including fatalities. | ||
:*''Metabolism and nutrition disorders'': [[hyponatremia]] | :*''Metabolism and nutrition disorders'': [[hyponatremia]] | ||
:*''Skin and subcutaneous tissue disorders'': Occurrences of [[Stevens-Johnson Syndrome]] (SJS) and [[toxic epidermal necrolysis]] (TEN), including fatal cases, have been reported in patients who were receiving or had recently been treated with Clofarabine and other medications (e.g., allopurinol or antibiotics) known to cause these syndromes. Other exfoliative conditions have also been reported. | :*''Skin and subcutaneous tissue disorders'': Occurrences of [[Stevens-Johnson Syndrome]] (SJS) and [[toxic epidermal necrolysis]] (TEN), including fatal cases, have been reported in patients who were receiving or had recently been treated with Clofarabine and other medications (e.g., [[allopurinol]] or [[antibiotics]]) known to cause these syndromes. Other exfoliative conditions have also been reported. | ||
|drugInteractions=* No in-vivo drug interaction studies have been conducted | |drugInteractions=* No in-vivo drug interaction studies have been conducted | ||
|useInPregnancyFDA='''Pregnancy Category D''' | |useInPregnancyFDA='''Pregnancy Category D''' | ||
Revision as of 15:25, 6 March 2015
{{DrugProjectFormSinglePage |authorTag=Kiran Singh, M.D. [1]; Sree Teja Yelamanchili, MBBS [2] |genericName=clofarabine |aOrAn=an |drugClass=antineoplastic agent |indicationType=treatment |indication=relapsed or refractory acute lymphoblastic leukemia after at least two prior regimens |adverseReactions=hypotension, tachycardia, abdominal pain,diarrhea, anemia, lymphocytopenia, thrombocytopenia, headache, anxiety, epistaxis, fatigue |blackBoxWarningTitle=ConditionName: |blackBoxWarningBody=ConditionName:
- Content
|fdaLIADAdult=There is limited information regarding Guideline-Supported Use of Clofarabine in adult patients.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Clofarabine in adult patients.
|offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Clofarabine in adult patients.
|fdaLIADPed=* Clofarabine Injection is indicated for the treatment of pediatric patients 1 to 21 years old with relapsed or refractory acute lymphoblastic leukemia after at least two prior regimens. This indication is based upon response rate. There are no trials verifying an improvement in disease-related symptoms or increased survival with Clofarabine.
Dosage
- Administer the recommended pediatric dose of 52 mg/m2 as an intravenous infusion over 2 hours daily for 5 consecutive days.
- Treatment cycles are repeated following recovery or return to baseline organ function, approximately every 2 to 6 weeks. The dosage is based on the patient's body surface area (BSA), calculated using the actual height and weight before the start of each cycle. To prevent drug incompatibilities, no other medications should be administered through the same intravenous line.
- Provide supportive care, such as intravenous fluids, antihyperuricemic treatment, and alkalinize urine throughout the 5 days of Clofarabine administration to reduce the effects of tumor lysis and other adverse events.
- Discontinue Clofarabine if hypotension develops during the 5 days of administration.
- Reduce the dose by 50% in patients with creatinine clearance (CrCL) between 30 and 60 mL/min. There is insufficient information to make a dosage recommendation in patients with CrCL less than 30 mL/min.
Dose Modifications and Reinitiation of Therapy
Hematologic Toxicity
- Administer subsequent cycles no sooner than 14 days from the starting day of the previous cycle and provided the patient's ANC is ≥ 0.75 × 109/L.
- If a patient experiences a Grade 4 neutropenia (ANC <0.5 × 109/L) lasting ≥4 weeks, reduce dose by 25% for the next cycle.
Non-hematologic Toxicity
- Withhold Clofarabine if a patient develops a clinically significant infection, until the infection is controlled, then restart at the full dose.
- Withhold Clofarabine for a Grade 3 non-infectious non-hematologic toxicity (excluding transient elevations in serum transaminases and/or serum bilirubin and/or nausea/vomiting controlled by antiemetic therapy). Re-institute Clofarabine administration at a 25% dose reduction when resolution or return to baseline.
- Discontinue Clofarabine administration for a Grade 4 non-infectious non-hematologic toxicity.
- Discontinue Clofarabine administration if a patient shows early signs or symptoms of SIRS or capillary leak (e.g., hypotension, tachycardia, tachypnea, and pulmonary edema) occur and provide appropriate supportive measures.
- Discontinue Clofarabine administration if Grade 3 or higher increases in creatinine or bilirubin are noted. Re-institute Clofarabine with a 25% dose reduction, when the patient is stable and organ function has returned to baseline. If hyperuricemia is anticipated (tumor lysis), initiate measures to control uric acid.
DOSAGE FORMS AND STRENGTHS
- 20 mg/20 mL (1 mg/mL) single-use vial
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Clofarabine in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Clofarabine in pediatric patients. |contraindications=* None |warnings=Myelosuppression
- Clofarabine causes myelosuppression which may be severe and prolonged. Febrile neutropenia occurred in 55% and non-febrile neutropenia in an additional 10% of pediatric patients in clinical trials. At initiation of treatment, most patients in the clinical studies had hematological impairment as a manifestation of leukemia. Myelosuppression is usually reversible with interruption of Clofarabine treatment and appears to be dose-dependent. Monitor complete blood counts.
Hemorrhage
- Serious and fatal hemorrhage, including cerebral, gastrointestinal and pulmonary hemorrhage, has occurred. The majority of the cases were associated with thrombocytopenia. Monitor platelets and coagulation parameters and treat accordingly.
Infections
- Clofarabine increases the risk of infection, including severe and fatal sepsis, and opportunistic infections. At baseline, 48% of the pediatric patients had one or more concurrent infections. A total of 83% of patients experienced at least one infection after Clofarabine treatment, including fungal, viral and bacterial infections. Monitor patients for signs and symptoms of infection, discontinue Clofarabine, and treat promptly.
Hyperuricemia (Tumor Lysis)
- Administration of Clofarabine may result in tumor lysis syndrome associated with the break-down metabolic products from peripheral leukemia cell death. Monitor patients undergoing treatment for signs and symptoms of tumor lysis syndrome and initiate preventive measures including adequate intravenous fluids and measures to control uric acid.
Systemic Inflammatory Response Syndrome (SIRS) and Capillary Leak Syndrome
- Clofarabine may cause a cytokine release syndrome (e.g., tachypnea, tachycardia, hypotension, pulmonary edema) that may progress to the systemic inflammatory response syndrome (SIRS) with capillary leak syndrome and organ impairment which may be fatal. Monitor patients frequently for these conditions. In clinical trials, SIRS was reported in two patients (2%); capillary leak syndrome was reported in four patients (4%). Symptoms included rapid onset of respiratory distress, hypotension, pleural and pericardial effusion, and multi-organ failure. Close monitoring for this syndrome and early intervention may reduce the risk. Immediately discontinue Clofarabine and provide appropriate supportive measures. The use of prophylactic steroids (e.g., 100 mg/m2 hydrocortisone on Days 1 through 3) may be of benefit in preventing signs or symptoms of SIRS or capillary leak. Consider use of diuretics and/or albumin. After the patient is stabilized and organ function has returned to baseline, re-treatment with Clofarabine can be considered with a 25% dose reduction.
Venous Occlusive Disease of the Liver
- Patients who have previously received a hematopoietic stem cell transplant (HSCT) are at higher risk for veno-occlusive disease (VOD) of the liver following treatment with clofarabine (40 mg/m2) when used in combination with etoposide (100 mg/m2) and cyclophosphamide (440 mg/m2). Severe hepatotoxic events have been reported in a combination study of clofarabine in pediatric patients with relapsed or refractory acute leukemia. Two cases (2%) of VOD in the mono-therapy studies were considered related to study drug. Monitor for and discontinue Clofarabine if VOD is suspected.
Hepatotoxicity
- Severe and fatal hepatotoxicity has occurred with the use of Clofarabine. In clinical studies, Grade 3–4 liver enzyme elevations were observed in pediatric patients during treatment with Clofarabine at the following rates: elevated aspartate aminotransferase (AST) occurred in 36% of patients; elevated alanine aminotransferase (ALT) occurred in 44% of patients. AST and ALT elevations typically occurred within 10 days of Clofarabine administration and returned to Grade 2 or less within 15 days. Grade 3 or 4 elevated bilirubin occurred in 13% of patients, with 2 events reported as Grade 4 hyperbilirubinemia (2%), one of which resulted in treatment discontinuation and one patient had multi-organ failure and died. Eight patients (7%) had Grade 3 or 4 elevations in serum bilirubin at the last time point measured; these patients died due to sepsis and/or multi-organ failure. Monitor hepatic function and discontinue Clofarabine for Grade 3 or greater liver enzyme elevations.
Renal Toxicity
- In clinical studies, Grade 3 or 4 elevated creatinine occurred in 8% of patients; acute renal failure was reported as Grade 3 in three patients (3%) and Grade 4 in two patients (2%). Hematuria was observed in 13% of patients overall. Monitor patients for renal toxicity and interrupt or discontinue Clofarabine as necessary.
Enterocolitis
- Fatal and serious cases of enterocolitis, including neutropenic colitis, cecitis, and C. difficile colitis, have occurred during treatment with clofarabine. This has occurred more frequently within 30 days of treatment, and in the setting of combination chemotherapy. Enterocolitis may lead to necrosis, perforation, hemorrhage or sepsis complications . Monitor patients for signs and symptoms of enterocolitis and treat promptly.
Skin Reactions
- Serious and fatal cases of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported. Discontinue Clofarabine for exfoliative or bullous rash, or if SJS or TEN is suspected.
Embryo-fetal Toxicity
- Clofarabine can cause fetal harm when administered to a pregnant woman. Intravenous doses of clofarabine in rats and rabbits administered during organogenesis caused an increase in resorptions, malformations, and variations
|clinicalTrials=* The following adverse reactions are discussed in greater detail in other sections of the label:
- Myelosuppression
- Hemorrhage
- Serious Infections
- Hyperuricemia (Tumor Lysis)
- Systemic Inflammatory Response Syndrome (SIRS) and Capillary Leak Syndrome
- Venous Occlusive Disease of the Liver
- Hepatotoxicity
- Renal Toxicity
- Enterocolitis
- Skin Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described below reflect exposure to Clofarabine in 115 pediatric patients with relapsed or refractory Acute Lymphoblastic Leukemia (ALL) (70 patients) or Acute Myelogenous Leukemia (AML) (45 patients).
- In total, 115 pediatric patients treated in clinical trials received the recommended dose of Clofarabine 52 mg/m2 daily × 5. The median number of cycles was 2. The median cumulative amount of Clofarabine received by pediatric patients during all cycles was 540 mg.
- The most common adverse reactions occurring in 10% or more of patients treated with Clofarabine are: nausea, vomiting, diarrhea, febrile neutropenia, headache, rash, pruritus, pyrexia, fatigue, palmar-plantar erythrodysesthesia syndrome, anxiety, flushing, and mucosal inflammation.
- Table 1 lists adverse reactions by System Organ Class, including severe or life-threatening (NCI CTC Grade 3 or Grade 4), reported in ≥ 5% of the 115 patients in the 52 mg/m2/day dose group (pooled analysis of pediatric patients with ALL and AML). More detailed information and follow-up of certain events is given below.
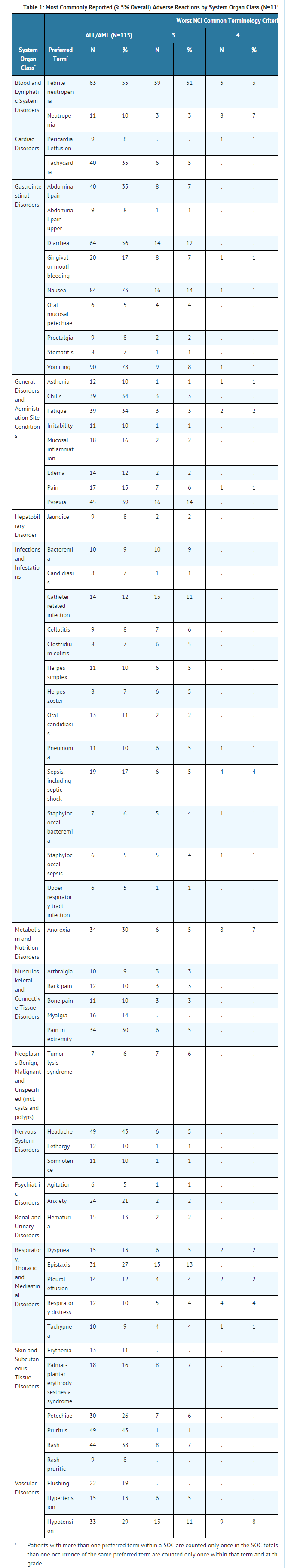
- The following less common adverse reactions have been reported in 1–4% of the 115 pediatric patients with ALL or AML:
- Gastrointestinal Disorders: cecitis, pancreatitis
- Hepatobiliary Disorders: hyperbilirubinemia
- Immune System Disorders: hypersensitivity
- Infections and Infestations: bacterial infection, Enterococcal bacteremia, Escherichia bacteremia, Escherichia sepsis, fungal infection, fungal sepsis, gastroenteritis adenovirus, infection, influenza, parainfluenza virus infection, fungal pneumonia, pneumonia primary atypical, Template:RSV
{{#subobject:
|Page Name=Clofarabine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Clofarabine |Label Name=Clofarabine image.jpg
}}
{{#subobject:
|Label Page=Clofarabine |Label Name=Clofarabine ingredients and appearance.png
}}