Chorionic Gonadotropin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Chorionic Gonadotropin is a gonadotropin that is FDA approved for the treatment of prepubertal cryptorchidism not due to anatomic obstruction, hypogonadotropic hypogonadism, anovulatory, infertile woman in whom the cause of anovulation is secondary and not due to primary ovarian failure, and who has been appropriately pretreated with human menotropins. Common adverse reactions include injection site pain, gynecomastia, precocious puberty, headache, depression, irritability, restlessness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- HCG HAS NOT BEEN DEMONSTRATED TO BE EFFECTIVE ADJUNCTIVE THERAPY IN THE TREATMENT OF OBESITY. THERE IS NO SUBSTANTIAL EVIDENCE THAT IT INCREASES WEIGHT LOSS BEYOND THAT RESULTING FROM CALORIC RESTRICTION, THAT IT CAUSES A MORE ATTRACTIVE OR "NORMAL" DISTRIBUTION OF FAT, OR THAT IT DECREASES THE HUNGER AND DISCOMFORT ASSOCIATED WITH CALORIE-RESTRICTED DIETS.
- Prepubertal cryptorchidism not due to anatomic obstruction. In general, HCG is thought to induce testicular descent in situations when descent would have occurred at puberty. HCG thus may help to predict whether or not orchiopexy will be needed in the future. Although, in some cases, descent following HCG administration is permanent, in most cases the response is temporary. Therapy is usually instituted between the ages of 4 and 9.
- Selected cases of hypogonadotropic hypogonadism (hypogonadism secondary to a pituitary deficiency) in males.
- Induction of ovulation and pregnancy in the anovulatory, infertile woman in whom the cause of anovulation is secondary and not due to primary ovarian failure, and who has been appropriately pretreated with human menotropins.
Dosage
- (Intramuscular Use Only): The dosage regimen employed in any particular case will depend upon the indication for use, the age and weight of the patient, and the physician's preference. The following regimens have been advocated by various authorities.
- Prepubertal cryptorchidism not due to anatomical obstruction:
- 4,000 USP Units three times weekly for three weeks.
- 5,000 USP Units every second day for four injections.
- 15 injections of 500 to 1,000 USP Units over a period of six weeks.
- 500 USP Units three times weekly for four to six weeks. If this course of treatment is not successful, another is begun one month later, giving 1,000 USP Units per injection.
- Selected cases of hypogonadotropic hypogonadism in males:
- 500 to 1,000 USP Units three times a week for three weeks, followed by the same dose twice a week for three weeks.
- 4,000 USP Units three times weekly for six to nine months, following which the dosage may be reduced to 2,000 USP Units three times weekly for an additional three months.
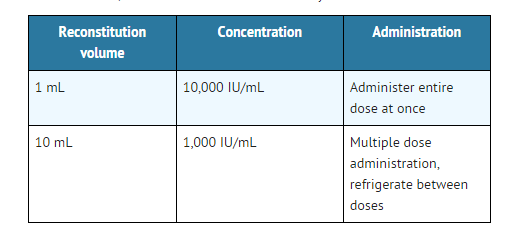
This image is provided by the National Library of Medicine. - Induction of ovulation and pregnancy in the anovulatory, infertile woman in whom the cause of anovulation is secondary and not due to primary ovarian failure and who has been appropriately pretreated with human menotropins.
- 5,000 to 10,000 USP Units one day following the last dose of menotropins. (A dosage of 10,000 USP Units is recommended in the labeling for menotropins).
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Disposing of Needles and Syringes
- To safely dispose of medical sharps, place used needles and syringes in a closeable, puncture-resistant container, such as a red biohazard sharps container. Sharps containers should then be taken to a collection center for proper disposal. Ask your physician or pharmacist or reference our website for more information about safely disposing used sharps.
- In some states, it is illegal to throw away medical sharps in household garbage, recycling, and compost bins. Needles and other sharps must be placed in an approved sharps container and disposed of at an approved drop-off site.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chorionic Gonadotropin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chorionic Gonadotropin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Chorionic Gonadotropin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chorionic Gonadotropin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chorionic Gonadotropin in pediatric patients.
Contraindications
- Precocious puberty, prostatic carcinoma or other androgen-dependent neoplasm, prior allergic reaction to HCG. HCG may cause fetal harm when administered to a pregnant woman. Combined HCG/PMS (pregnant mare's serum) therapy has been noted to induce high incidences of external congenital anomalies in the offspring of mice, in a dose-dependent manner. The potential extrapolation to humans has not been determined.
Warnings
- HCG should be used in conjunction with human menopausal gonadotropins only by physicians experienced with infertility problems who are familiar with the criteria for patient selection, contraindications, warnings, precautions, and adverse reactions described in the package insert for menotropins. The principal serious adverse reactions during this use are: (1) Ovarian hyperstimulation, a syndrome of sudden ovarian enlargement, ascites with or without pain, and/or pleural effusion; (2) Enlargement of preexisting ovarian cysts or rupture of ovarian cysts with resultant hemoperitoneum; (3) Multiple births, and (4) Arterial thromboembolism.
- The recommended diluent for reconstitution is Bacteriostatic Water for Injection preserved with benzyl alcohol 0.9%. Benzyl alcohol has been reported to be associated with a fatal "Gasping Syndrome" in premature infants.
- Anaphylaxis has been reported with urinary-derived HCG products.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Chorionic Gonadotropin Clinical Trials Experience in the drug label.
Postmarketing Experience
- Headache, irritability, restlessness, depression, fatigue, edema, precocious puberty, gynecomastia, pain at the site of injection. Hypersensitivity reactions both localized and systemic in nature, including erythema, urticaria, rash, angioedema, dyspnea and shortness of breath, have been reported. The relationship of these allergic-like events to the polypeptide hormone or the diluent containing benzyl alcohol is not clear.
Drug Interactions
There is limited information regarding Chorionic Gonadotropin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Combined HCG/PMS (pregnant mare's serum) therapy has been noted to induce high incidences of external congenital anomalies in the offspring of mice, in a dose-dependent manner. The potential extrapolation to humans has not been determined.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chorionic Gonadotropin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chorionic Gonadotropin during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when HCG is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children below the age of 4 have not been established.
Geriatic Use
There is no FDA guidance on the use of Chorionic Gonadotropin in geriatric settings.
Gender
There is no FDA guidance on the use of Chorionic Gonadotropin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chorionic Gonadotropin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chorionic Gonadotropin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Chorionic Gonadotropin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chorionic Gonadotropin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chorionic Gonadotropin in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Chorionic Gonadotropin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Chorionic Gonadotropin and IV administrations.
Overdosage
There is limited information regarding Chorionic Gonadotropin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- The action of HCG is virtually identical to that of pituitary LH, although HCG appears to have a small degree of FSH activity as well. It stimulates production of gonadal steroid hormones by stimulating the interstitial cells (Leydig cells) of the testis to produce androgens and the corpus luteum of the ovary to produce progesterone. Androgen stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present. This descent is usually reversible when HCG is discontinued. During the normal menstrual cycle, LH participates with FSH in the development and maturation of the normal ovarian follicle, and the mid-cycle LH surge triggers ovulation. HCG can substitute for LH in this function.
- During a normal pregnancy, HCG secreted by the placenta maintains the corpus luteum after LH secretion decreases, supporting continued secretion of estrogen and progesterone, and preventing menstruation. HCG HAS NO KNOWN EFFECT ON FAT MOBILIZATION, APPETITE OR SENSE OF HUNGER, OR BODY FAT DISTRIBUTION.
Structure
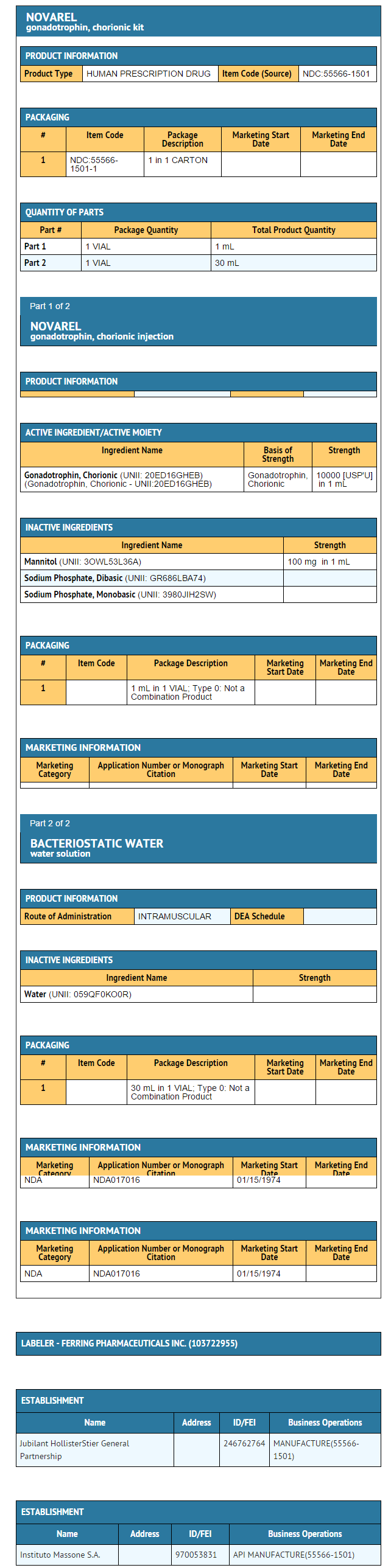
- Human chorionic gonadotropin (HCG), a polypeptide hormone produced by the human placenta, is composed of an alpha and a beta subunit. The alpha subunit is essentially identical to the alpha subunits of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha subunit of human thyroid-stimulating hormone (TSH). The beta subunits of these hormones differ in amino acid sequence.
- Chorionic Gonadotropin is a water soluble glycoprotein derived from human pregnancy urine. The sterile lyophilized powder is stable. When reconstituted with Bacteriostatic Water for Injection preserved with benzyl alcohol 0.9%, the solution should be refrigerated and used within 30 days.
- Each vial contains:
- Chorionic Gonadotropin 10,000 USP Units, Mannitol 100 mg, Dibasic Sodium Phosphate 16 mg, and Monobasic Sodium Phosphate 4 mg.
Pharmacodynamics
There is limited information regarding Chorionic Gonadotropin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Chorionic Gonadotropin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Chorionic Gonadotropin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Chorionic Gonadotropin Clinical Studies in the drug label.
How Supplied
- Chorionic Gonadotropin for Injection, USP, is available as individually packaged vials containing 10,000 USP Units per vial .
- Each vial of Novarel® is accompanied by a vial of sterile diluent containing 30 mL of bacteriostatic water for Injection USP, containing 0.9% benzyl alcohol.
- 10,000 USP Units of Chorionic Gonadotropin injection, supplied as:
NDC 55566-1501-1: Box of 1 vial + 1 vial diluent.
Storage
- Store dry product at 20° to 25°C (68° to 77°F), excursions permitted between 15° and 30°C (between 59° and 86°F)
Images
Drug Images
{{#ask: Page Name::Chorionic Gonadotropin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Chorionic Gonadotropin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Chorionic Gonadotropin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Chorionic Gonadotropin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- NOVAREL ®[1]
Look-Alike Drug Names
There is limited information regarding Chorionic Gonadotropin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.