Chondrocalcinosis
| Chondrocalcinosis | |
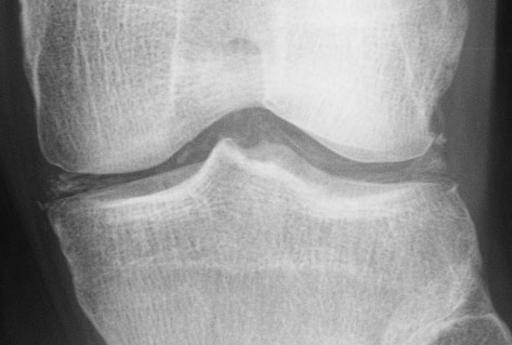 | |
|---|---|
| Chondrocalcinosis of the articular and fibrocartilage of the left knee in a patient with calcium pyrophosphate dihydrate deposition disease (CPPD) | |
| ICD-10 | M11.1-M11.2 |
| ICD-9 | 712.3 |
| DiseasesDB | 10832 |
| MedlinePlus | 000421 |
| MeSH | D002805 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [2] Luke Rusowicz-Orazem, B.S. Mohammed Abdelwahed M.D[3]
Synonyms and keywords:: Pyrophosphate arthropathy; chondrocalcinosis; pseudogout; CPPD; Calcium pyrophosphate dihydrate deposition disease; CPPD disease
Overview
Calcium pyrophosphate deposition disease (CPPD) is a rheumatologic disorder with varied clinical manifestations due to precipitation of calcium pyrophosphate dihydrate crystals in the connective tissues. It is more commonly known by alternative names that specify certain clinical or radiographic findings, although neither is synonymous with CPPD. Pseudogout refers to the clinically evident acute synovitis with red, tender, and swollen joints that may resemble gouty arthritis (a similar condition with joint deposition of monosodium urate crystals). Chondrocalcinosis, on the other hand, refers to the radiographic evidence of calcification in hyaline and/or fibrocartilage. Pyrophosphate arthropathy is a term that may refer to either of the above, but is uncommonly used.
Causes
Life Threatening Causes
Causes in Alphabetical Order
Epidemiology
- Calcium pyrophosphate crystal deposition (CPPD) disease has been estimated to affect 4 to 7 percent of the adult populations of Europe and the United States. [7,8]
- The average age at diagnosis of CPPD disease in one study was 72 years [3].
- The prevalence of radiographic calcium pyrophosphate deposition according to age was:
65 to 74 years – 15 percent
75 to 84 years – 36 percent
>84 years – Almost 50 percent
- There is no major gender predominance in CPPD. Attacks of acute arthritis may occur more frequently in men.
Pathogenesis
- Calcium pyrophosphate crystal formation reflects elevated levels of calcium or inorganic pyrophosphate in cartilages matrix.
- Excessive pyrophosphate levels arises from nucleoside triphosphate pyrophosphohydrolase enzyme overactivity.
- Increased concentrations of substrate (adenosine triphosphate) for this enzyme are also present in joint fluids from persons with CPPD.
- ANKH gene plays a role in formation of the crystals. There is now evidence that the ANKH gene product also promotes the release of ATP by chondrocytes. [14]
- One function of extracellular pyrophosphate appears to be to bind to and inhibit the growth of basic calcium phosphate crystals.
- In CPPD, excessive pyrophosphate levels arising from NTPPPH overactivity could provide the substrate for CPP crystal formation in the immediate environment of chondrocytes.
- Mutations in or just upstream of the chromosome 5p locus of ANKH have also been identified in some individuals with idiopathic or sporadic CPPD deposition disease.
- Degenerative arthritis accompanying CPPD frequently involves such joints as the metacarpophalangeal and wrist joints, which are commonly spared in classical osteoarthritis.
Clinical presentation
- Asymptomatic CPPD disease: Most joints in which CPP crystal deposition is readily apparent on radiographs are asymptomatic, even among patients in whom acute or chronic clinical manifestations of CPPD disease in one or several other joints have occurred.
- Acute CPP crystal arthritis: The knee is affected in over 50 percent of all acute attacks of acute CPP crystal arthritis. Other joints typically affected in acute CPP crystal arthritis include wrists, shoulders, ankles, feet, and elbows. Predisposing factors include trauma, surgery, or severe medical illness often provoke acute attacks. Treatment with pamidronate or granulocyte-macrophage-colony-stimulating factor have also been reported to precipitate acute attacks of pseudogout.
- Chronic CPP crystal inflammatory arthritis: the chronic inflammatory arthritis of CPPD disease involves multiple joints, frequently involving peripheral joints of the upper and lower extremities, including the wrists and metacarpophalangeal joints, as well as the knees and elbows, in a symmetric or nearly symmetric pattern. Chronic CPP crystal inflammatory arthritis occurs in 5 percent or less of patients with symptomatic CPPD disease.
- Severe joint degeneration: Neuropathic arthropathy is characterized by severe joint degeneration and disruption occurring in the course of neurologic disorders leading to joint denervation; the affected joint is often called a Charcot joint. Underlying disorders associated with Charcot joints include diabetes mellitus (most common), tabes dorsalis, and syringomyelia.
- Spinal involvement: CPP crystal deposition in and about the spine has been associated with a number of clinical manifestations, including spine stiffness, sometimes associated with bony ankylosis, which can resemble the spinal changes of ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis.
Diagnosis
- Synovial fluid: Identification of CPP crystals is diagnostic. Successful identification of the crystals diminishes as the time between joint aspiration and microscopic examination increases.
Radiological findings
Three main manifestations of CPPD deposition:
- chondrocalcinosis
- crystal induced synovitis
- pyrophosphate arthropathy
Plain film radiography
- Cartilage: CPP crystal deposits in hyaline cartilage frequently appear as a radiopaque line paralleling the surface of the underlying bone.
- Joints: Larger joints are frequently involved in CPPD disease. Synovial calcification is often fainter and more diffuse than cartilage calcification. Linear calcifications involving the Achilles tendon or plantar fascia. [32].
Degenerative changes
- CPP crystal deposition is often associated with degenerative changes in joints. It includes subchondral cysts, osteophyte formation, and bone and cartilage fragmentation.
- Radiographic features of osteoarthritis
- Stress fractures or osteonecrosis (avascular necrosis)
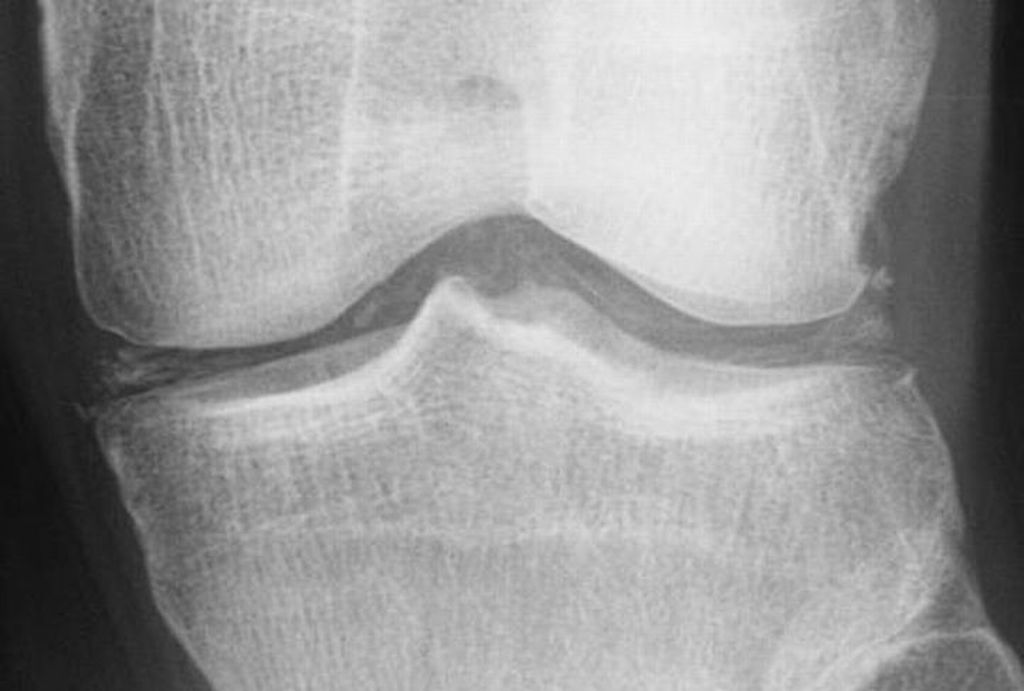
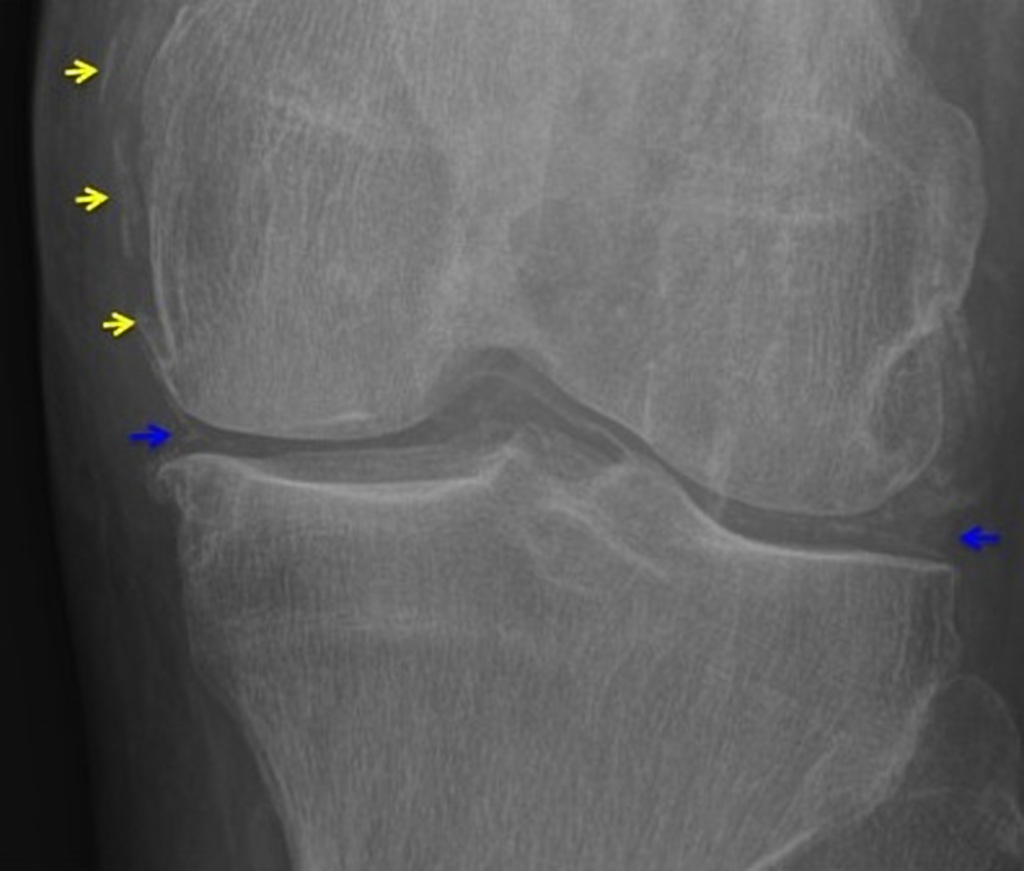
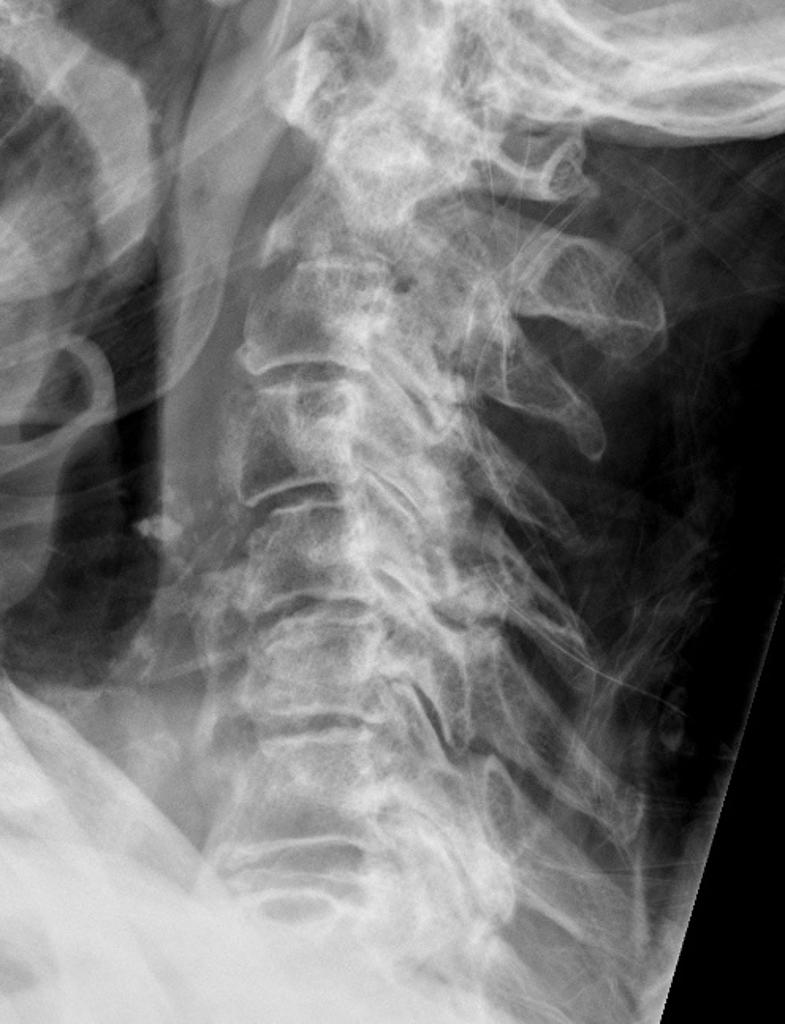
Ultrasonographic findings
The following findings on ultrasonography of articular and fibrocartilage:
- A thin hyperechoic band paralleling the bone cortex and separated from it by a hypoechoic region representing cartilage. The resulting ultrasonographic appearance resembles the double contour sign (DCS) initially described in gout, but it often exhibits a thin, stippled appearance rather than the smooth pattern characteristic of gout.
- Small hyperechoic rounded amorphous shaped regions, often with acoustic shadowing, which are most often found in images of fibrocartilage of the wrist (image 2) and menisci of the knee, and in tendons.
- Nodular hyperechoic deposits in bursae and articular recesses.
- Hyperechoic lines of calcification running parallel to tendon fibers.
Differential diagnosis
Acute arthritis
Pseudogout acute attacks should be differentiated from other causes of acute monoarthritis such as:
- Fracture
- Osteonecrosis
- Meniscal tear
- Plant thorn synovitis
Chronic inflammatory arthritis
Chronic CPP inflammatory arthritis should be differentiated from other forms of chronic inflammatory arthritis such as:
- Rheumatoid arthritis
- Spondyloarthritis
- Systemic lupus erythematosus
- Sarcoidosis
- Osteoarthritis
- Peripheral spondyloarthritis, including reactive arthritis and arthritis associated with inflammatory bowel disease, as well as psoriatic arthritis.
Tumors such as:
- Tenosynovial giant cell tumor
- Chondrosarcoma
- Osteoid osteoma
Treatment
- Treatment of CPPD is mostly aimed at preventing further crystal formation and reducing symptoms from crystal deposition. CPPD crystal deposition cannot be reversed. If CPPD results from underlying metabolic abnormalities (hyperparathyroidism, hemochromatosis, hypophosphatasia, or hypomagnesemia)[1], these can be treated directly.
- Treatment is similar to the treatment of gout. When a single joint is involved, joint aspiration and intra-articular corticosteroid injection is often used, in addition to NSAIDs and/or colchicine. When multiple joints are involved, joint injection is often impractical, or limited only to the most severely involved joint, and oral systemic treatment is chosen instead.
- Because pyrophosphate complexes with magnesium prior to its degradation [2], and CPPD can result from pathologically low magnesium levels, magnesium supplementation may be of help, particularly in a few patients with underlying hypomagnesemia. It may be possible to end attacks by ingesting large (maximum RDA) doses of magnesium supplement accompanied by vitamin B6 to help absorption. What that does is assist the body in re-dissolving the calcium in the joint fluid, and with maintenance doses, often in the form of snacking on magnesium-rich foods such as almonds, further attacks can be prevented altogether - without medication.
- Anti-inflammatory medication, usually by intraarticular injection, supported by local measures to reduce symptoms:
- Joint fluid aspiration and glucocorticoid injection usually provide relief in pain and swelling within 8 to 24 hours.
- application of ice or cool packs and immobilization and resting of the joint by restriction of weight bearing.
- systemic anti-inflammatory therapy is warranted, employing agents typically used for the treatment of acute gout.
- These include: NSAIDs, which may, however, be contraindicated in older adults, the population especially susceptible to acute attacks of pseudogout;
- colchicine, which is particularly useful if a low-dose regimen is initiated within hours of attack onset;
- glucocorticoids, which are efficacious in patients unable to take NSAIDs or colchicine.
- In patients in whom treatment for acute CPP crystal arthritis is initiated within about 24 hours of onset and whose clinical and concomitant medication profiles permit its consideration, we suggest oral colchicine in a low-dose regimen as initially recommended for the treatment of acute gout, rather than using oral glucocorticoids [7,9]. For regimens using low-dose colchicine, no more than 1.8 mg of colchicine is taken orally in the first 24 hours of treatment, followed by 0.6 mg colchicine taken twice daily until the attack abates [9]. This strategy is based upon a treatment regimen for acute gout and not on studies of acute CPP crystal arthritis.
- Common adverse effects of colchicine may include diarrhea and abdominal cramping, but these are less likely in patients who receive no more than 1.8 mg in total on the first day compared with patients receiving higher doses, such as 0.6 mg every one to two hours until symptom relief or intolerance (as was historically employed) [11].
- In patients in whom glucocorticoid therapy is inadequate, it is important to confirm the absence of hepatic disease that might impair the conversion of prednisone to prednisolone, in which case the glucocorticoid therapy can be switched to oral prednisolone.
- Since pseudogout attacks are self-limited, an alternative option is managing the symptoms using supportive measures until they resolve with analgesics, rest, splinting, and icing.
References
- ↑ Wright G.D., Doherty M. Calcium pyrophosphate crystal deposition is not always 'wear and tear' or aging. Ann Rheum Dis.1997; 56: 586-588 PMID 9389218
- ↑ Wright G.D., Doherty M. Calcium pyrophosphate crystal deposition is not always 'wear and tear' or aging. Ann Rheum Dis.1997; 56: 586-588 PMID 9389218