Chlorothiazide (oral suspension): Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 6: | Line 6: | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=[[edema]] and [[hypertention]] | |indication=[[edema]] and [[hypertention]] | ||
|adverseReactions=[[ | |adverseReactions=[[weakness]], [[hypotension]], [[diarrhea]], [[vomiting]], [[nausea]], [[anorexia]] and | ||
[[thrombocytopenia]] | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
Revision as of 20:24, 11 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Chlorothiazide (oral suspension) is a diuretic that is FDA approved for the treatment of edema and hypertention. Common adverse reactions include weakness, hypotension, diarrhea, vomiting, nausea, anorexia and thrombocytopenia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- DIURIL is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
- DIURIL has also been found useful in edema due to various forms of renal dysfunction such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
- DIURIL is indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effectiveness of other antihypertensive drugs in the more severe forms of hypertension.
- Use in Pregnancy. Routine use of diuretics during normal pregnancy is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy and there is no satisfactory evidence that they are useful in the treatment of toxemia.
- Edema during pregnancy may arise from pathologic causes or from the physiologic and mechanical consequences of pregnancy. Thiazides are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy. Dependent edema in pregnancy, resulting from restriction of venous return by the gravid uterus, is properly treated through elevation of the lower extremities and use of support stockings. Use of diuretics to lower intravascular volume in this instance is illogical and unnecessary. During normal pregnancy there is hypervolemia which is not harmful to the fetus or the mother in the absence of cardiovascular disease. However, it may be associated with edema, rarely generalized edema. If such edema causes discomfort, increased recumbency will often provide relief. Rarely this edema may cause extreme discomfort which is not relieved by rest. In these instances, a short course of diuretic therapy may provide relief and be appropriate.
Dosage
- Therapy should be individualized according to patient response. Use the smallest dosage necessary to achieve the required response.
Adults
For Edema
- The usual adult dosage is 500 mg to 1000 mg (10 mL to 20 mL) once or twice a day. Many patients with edema respond to intermittent therapy, i.e., administration on alternate days or on three to five days each week. With an intermittent schedule, excessive response and the resulting undesirable electrolyte imbalance are less likely to occur.
For Control of Hypertension
- The usual adult starting dosage is 500 mg to 1000 mg (10 mL to 20 mL) a day as a single or divided dose. Dosage is increased or decreased according to blood pressure response. Rarely some patients may require up to 2000 mg (40 mL) a day in divided doses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorothiazide (oral suspension) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorothiazide (oral suspension) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Chlorothiazide (oral suspension) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chlorothiazide (oral suspension) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Chlorothiazide (oral suspension) in pediatric patients.
Contraindications
- Hypersensitivity to this product or to other sulfonamide-derived drugs.
Warnings
- Use with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
- Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
- Thiazides may add to or potentiate the action of other antihypertensive drugs.
- Sensitivity reactions may occur in patients with or without a history of allergy or bronchial asthma.
- The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
- Lithium generally should not be given with diuretics
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions have been reported and, within each category, are listed in order of decreasing severity.
- Body as a Whole: Weakness.
- Cardiovascular: Hypotension including orthostatic hypotension (may be aggravated by alcohol, barbiturates, narcotics or antihypertensive drugs).
- Digestive: Pancreatitis, jaundice (intrahepatic cholestatic jaundice), diarrhea, vomiting, sialadenitis, cramping, constipation, gastric irritation, nausea, anorexia.
- Hematologic: Aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia.
- Hypersensitivity: Anaphylactic reactions, necrotizing angiitis (vasculitis and cutaneous vasculitis), respiratory distress including pneumonitis and pulmonary edema, photosensitivity, fever, urticaria, rash, purpura.
- Metabolic: Electrolyte imbalance, hyperglycemia, glycosuria, hyperuricemia.
- Musculoskeletal: Muscle spasm.
- Nervous System/Psychiatric: Vertigo, paresthesias, dizziness, headache, restlessness.
- Skin: Erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis, alopecia.
- Special Senses: Transient blurred vision, xanthopsia.
- Urogenital: Impotence.
- Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Chlorothiazide (oral suspension) in the drug label.
Drug Interactions
- When given concurrently the following drugs may interact with thiazide diuretics.
- Alcohol, barbiturates, or narcotics - potentiation of orthostatic hypotension may occur.
- Antidiabetic drugs (oral agents and insulin) - dosage adjustment of the antidiabetic drug may be required.
- Other antihypertensive drugs - additive effect or potentiation.
- Cholestyramine and colestipol resins - Both cholestyramine and colestipol resins have the potential of binding thiazide diuretics and reducing diuretic absorption from the gastrointestinal tract.
- Corticosteroids, ACTH - intensified electrolyte depletion, particularly hypokalemia.
- Pressor amines (e.g., norepinephrine) - possible decreased response to pressor amines but not sufficient to preclude their use.
- Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine) - possible increased responsiveness to the muscle relaxant.
- Lithium - generally should not be given with diuretics. Diuretic agents reduce the renal clearance of lithium and add a high risk of lithium toxicity. Refer to the package insert for lithium preparations before use of such preparations with DIURIL.
- Non-steroidal Anti-inflammatory Drugs Including Selective Cyclooxygenase-2 (COX-2) Inhibitors - In some patients, the administration of a non-steroidal anti-inflammatory agent including a selective COX-2 inhibitor can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. Therefore, when DIURIL and non-steroidal anti-inflammatory agents or selective COX-2 inhibitors are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
- In some patients with compromised renal function (e.g., elderly patients or patients who are volume-depleted, including those on diuretic therapy) who are being treated with non-steroidal anti-inflammatory drugs, including selective COX-2 inhibitors, the co-administration of angiotensin II receptor antagonists or ACE inhibitors may result in a further deterioration of renal function, including possible acute renal failure. These effects are usually reversible.
- These interactions should be considered in patients taking NSAIDs including selective COX-2 inhibitors concomitantly with diuretics and angiotensin II antagonists or ACE inhibitors. Therefore, the combination should be administered with caution, especially in the elderly.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Teratogenic Effects - Pregnancy Category C: Although reproduction studies performed with chlorothiazide doses of 50 mg/kg/day in rabbits, 60 mg/kg/day in rats and 500 mg/kg/day in mice revealed no external abnormalities of the fetus or impairment of growth and survival of the fetus due to chlorothiazide, such studies did not include complete examinations for visceral and skeletal abnormalities. It is not known whether chlorothiazide can cause fetal harm when administered to a pregnant woman; however, thiazides cross the placental barrier and appear in cord blood. DIURIL should be used during pregnancy only if clearly needed.
Nonteratogenic Effects: Chlorothiazide may cause fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions which have occurred in the adult.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chlorothiazide (oral suspension) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chlorothiazide (oral suspension) during labor and delivery.
Nursing Mothers
- Because of the potential for serious adverse reactions in nursing infants from DIURIL, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- There are no well-controlled clinical trials in pediatric patients. Information on dosing in this age group is supported by evidence from empiric use in pediatric patients and published literature regarding the treatment of hypertension in such patients.
Geriatic Use
- Clinical studies of DIURIL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function
Gender
There is no FDA guidance on the use of Chlorothiazide (oral suspension) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chlorothiazide (oral suspension) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chlorothiazide (oral suspension) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Chlorothiazide (oral suspension) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chlorothiazide (oral suspension) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chlorothiazide (oral suspension) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Chlorothiazide (oral suspension) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Chlorothiazide (oral suspension) in the drug label.
Overdosage
- The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
- In the event of overdosage, symptomatic and supportive measures should be employed. Emesis should be induced or gastric lavage performed. Correct dehydration, electrolyte imbalance, hepatic coma and hypotension by established procedures. If required, give oxygen or artificial respiration for respiratory impairment.
- The degree to which chlorothiazide sodium is removed by hemodialysis has not been established.
- The oral LD50 of chlorothiazide is 8.5 g/kg, greater than 10 g/kg, and greater than 1 g/kg, in the mouse, rat and dog respectively.
Pharmacology
There is limited information regarding Chlorothiazide (oral suspension) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Chlorothiazide (oral suspension) Mechanism of Action in the drug label.
Structure
There is limited information regarding Chlorothiazide (oral suspension) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Chlorothiazide (oral suspension) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Chlorothiazide (oral suspension) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Chlorothiazide (oral suspension) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Chlorothiazide (oral suspension) in the drug label.
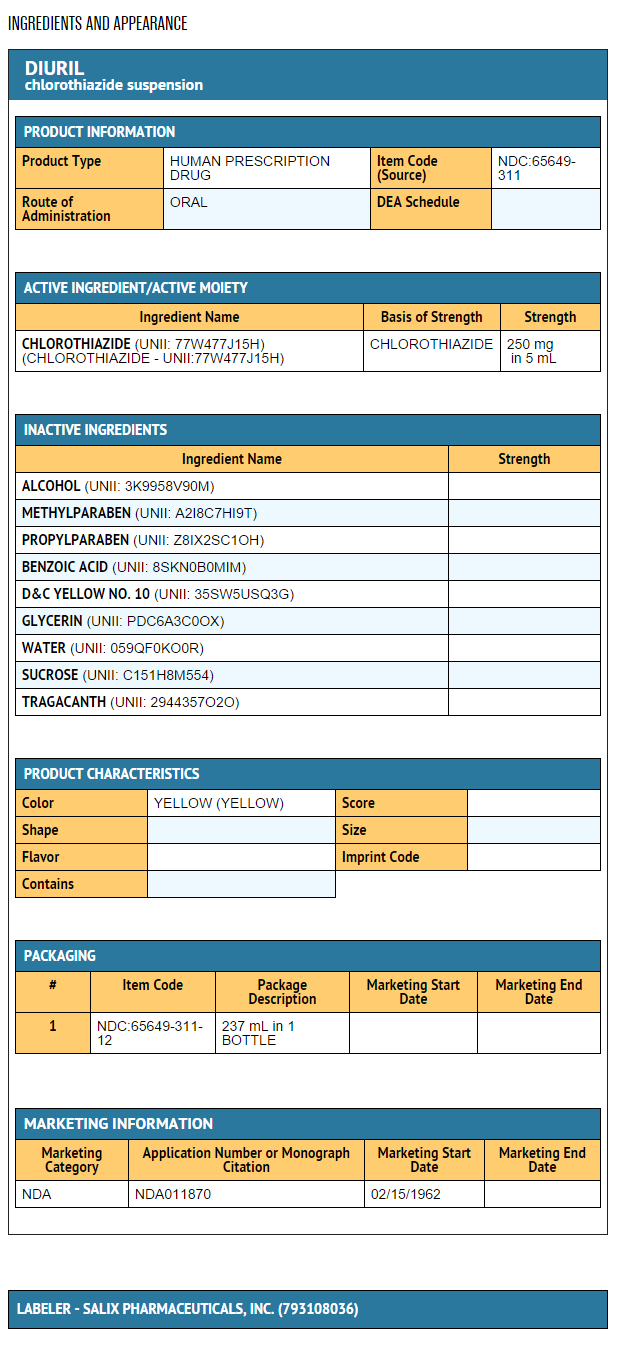
How Supplied
- DIURIL Oral Suspension, 250 mg of chlorothiazide per 5 mL, is a yellow, creamy suspension, and is supplied as follows:
- NDC 65649-311-12 bottles of 237 mL.
Storage
- DIURIL Oral Suspension: Keep container tightly closed. Protect from freezing, –20°C (–4°F) and store at room temperature, 15-30°C (59-86°F).
Images
Drug Images
{{#ask: Page Name::Chlorothiazide (oral suspension) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Chlorothiazide (oral suspension) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Chlorothiazide (oral suspension) in the drug label.
Precautions with Alcohol
- Alcohol-Chlorothiazide (oral suspension) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- DIURIL®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "chlorothiazide suspension".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Chlorothiazide (oral suspension)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Chlorothiazide (oral suspension) |Label Name=Chlorothiazide (oral suspension)11.png
}}
{{#subobject:
|Label Page=Chlorothiazide (oral suspension) |Label Name=Chlorothiazide (oral suspension)11.png
}}