Charcot-Marie-Tooth disease
| Charcot-Marie-Tooth disease | |
 | |
|---|---|
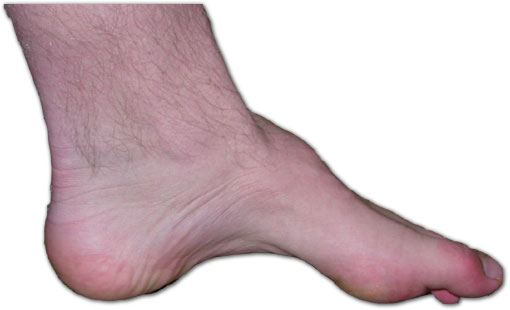
| The foot of a person with Charcot-Marie-Tooth. The lack of muscle, a high arch, and hammer toes are signs of the genetic disease. | |
| ICD-10 | G60.0 |
| ICD-9 | 356.1 |
| DiseasesDB | 5815 Template:DiseasesDB2 |
| MedlinePlus | 000727 |
| MeSH | D002607 |
|
Charcot-Marie-Tooth disease Microchapters |
|
Differentiating Charcot-Marie-Tooth disease from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Charcot-Marie-Tooth disease On the Web |
|
American Roentgen Ray Society Images of Charcot-Marie-Tooth disease |
|
Directions to Hospitals Treating Charcot-Marie-Tooth disease |
|
Risk calculators and risk factors for Charcot-Marie-Tooth disease |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: Hereditary motor and sensory neuropathy; peroneal muscular atrophy
Overview
Historical Perspective
Classification
Pathophysiology
Epidemiology and Demographics
Description
The disorder is caused by the absence of molecules that are essential for normal function of the nerves due to errors in the genes coding these molecules. The absence of these chemical substances gives rise to dysfunction either in the axon or the myelin sheath of the nerve cell. Most of the mutations identified result in disrupted myelin production, however the most common mutations occur in gene MFN2, which doesn't seem to have anything to do with myelin. Instead MFN2 controls behaviour of mitochondria. Recent research showed that the mutated MFN2 causes mitochondria to form large clusters. In nerve cells these large clusters of mitochondria failed to travel down the axon towards the synapses. It is suggested these mitochondria clots make the synapses fail, resulting in CMT disease.[1]
The different classes of this disorder have been divided into the primary demyelinating neuropathies (CMT1, CMT3, and CMT4) and the primary axonal neuropathies (CMT2). Recent studies, however, show that the pathologies of these two classes are frequently intermingled, due to the dependence and close cellular interaction of Schwann cells and neurons. Schwann cells are responsible for myelin formation, enwrapping neural axons with their plasma membranes in a process called “myelination”.[2]
The molecular structure of the nerve depends upon the interactions between neurons, Schwann cells, and fibroblasts. Schwann cells and neurons, in particular, exchange signals that regulate survival and differentiation during development. These signals are important to CMT disease because a disturbed communication between Schwann cells and neurons, resulting from a genetic defect, is observed in this disorder.[2]
It is clear that interaction with demyelinating Schwann cells causes the expression of abnormal axonal structure and function, but we still do not know how these abnormalities result in CMT. One possibility is that the weakness and sensory loss experienced by patients with CMT is a result of axonal degradation. Another possibility is that axonal dysfunction occurs, not degeneration, and that this dysfunction is induced by demyelinating Schwann cells.[3]
Most patients experience demyelinating neuropathies, and this is characterized by a reduction in nerve conduction velocity (NCV), due to a partial or complete loss of the myelin sheath. Axonopathies, on the other hand, are characterized by a reduced compound muscle action potential (CMAP), while NCV is normal or only slightly reduced.[2]
Diagnosis
The diagnosis is established by electromyography examination (which shows that the velocity of nerve impulse conduction is decreased and the time required to charge the nerve is increased) and nerve biopsy. Genetic markers have been identified for some, but not all forms of the disease.
Genetic testing
Genetic testing is available for many of the different types of Charcot-Marie-Tooth. For a listing of test availabilities, see GeneTests.org
External links
References
- ↑ Baloh, R., Schmidt, R., Pestronk, A. and Milbrandt, J. (2007) The Journal of Neuroscience 27(2):422-430, http://www.jneurosci.org/cgi/content/abstract/27/2/422 accessed 070122
- ↑ 2.0 2.1 2.2 Berger, P., Young, P. and U. Suter (2002) Neurogenetics 4:1-15. http://www.springerlink.com/, accessed 060220
- ↑ Krajewski, K.M., Lewis, R.A., Fuerst, D.R., Turansky, C., Hinderer, S.R., Gerbern, J., Kamholz, J. and M.E. Shy (2000) Brain 123:1516-1527 accessed 060220
Template:Muscular Dystrophy Template:PNS diseases of the nervous system
ca:Malaltia de Charcot-Marie-Tooth
de:Morbus Charcot-Marie-Tooth
it:Malattia di Charcot-Marie-Tooth
nl:Hereditaire Motorische en Sensorische Neuropathieën
no:Charcot-Marie-Tooths sykdom
sv:Charcot-Marie-Tooths sjukdom