Cetrorelix: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
||
| Line 26: | Line 26: | ||
==References== | ==References== | ||
{{reflist}} | {{reflist|2}} | ||
{{Sex hormones}} | {{Sex hormones}} | ||
Revision as of 15:26, 4 September 2012
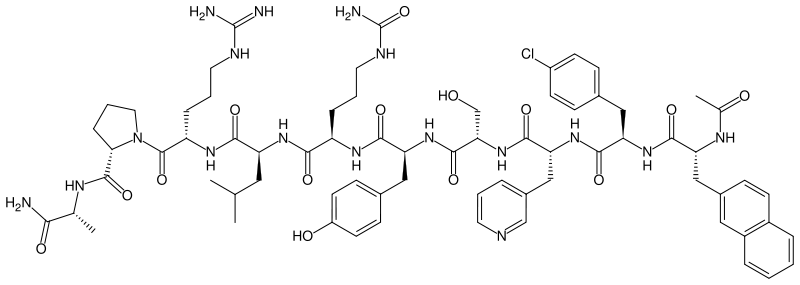 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Subcutaneous injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 86% |
| Elimination half-life | 62.8 hours / 3mg single dose |
| Excretion | Liver |
| Identifiers | |
| |
| CAS Number | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Molar mass | 1431.06 g/mol |
|
WikiDoc Resources for Cetrorelix |
|
Articles |
|---|
|
Most recent articles on Cetrorelix |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cetrorelix at Clinical Trials.gov Clinical Trials on Cetrorelix at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cetrorelix
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cetrorelix Discussion groups on Cetrorelix Patient Handouts on Cetrorelix Directions to Hospitals Treating Cetrorelix Risk calculators and risk factors for Cetrorelix
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cetrorelix |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Cetrorelix acetate is an injectable gonadotropin-releasing hormone antagonist (GnRH antagonist). A synthetic decapeptide, it is used to treat hormone-sensitive cancers of the prostate and breast (in pre-/perimenopausal women) and some benign gynaecological disorders (endometriosis, uterine fibroids and endometrial thinning). In addition, cetrorelix is used in assisted reproduction. The drug works by blocking the action of GnRH upon the pituitary, thus rapidly suppressing the production and action of LH and FSH. It is administered as a daily or weekly subcutaneous injection.
Cetrorelix is marketed by Solvay Pharmaceuticals as Cetrotide.[1]
References
Template:Sex hormones Template:Pituitary and hypothalamic hormones and analogues
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- GnRH antagonists
- Fertility medicine
- Endocrinology