Cethromycin
Jump to navigation
Jump to search
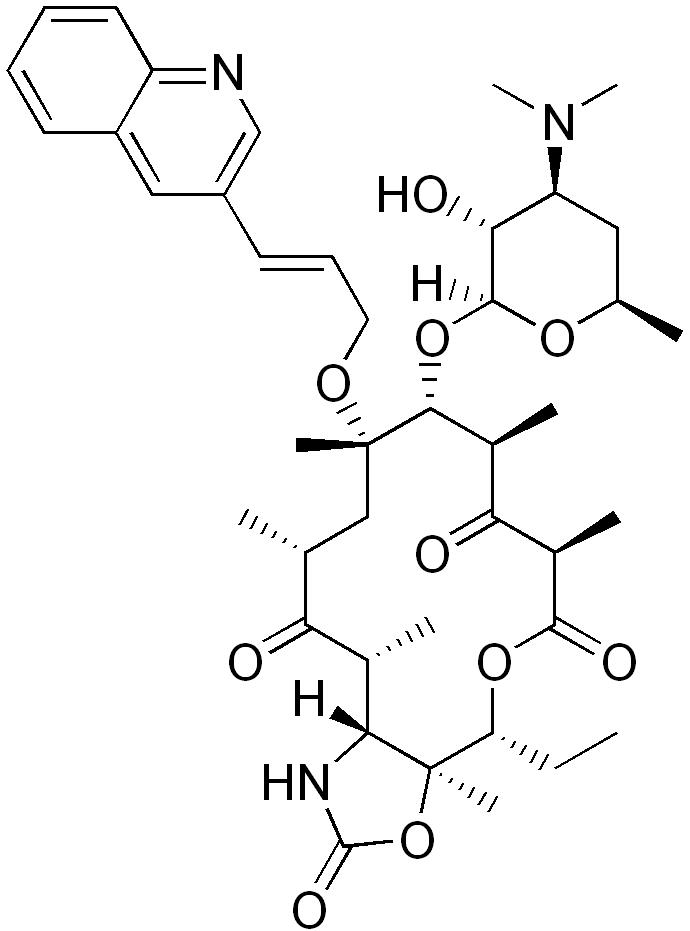 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C42H59N3O10 |
| Molar mass | 765.931 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Cethromycin (initially known as ABT-773) is a macrolide antibiotic undergoing research for the treatment of community acquired pneumonia (CAP) and for the prevention of post-exposure inhalational anthrax, and was given an "orphan drug" status for this indication.[1] Originally discovered and developed by Abbott, it was acquired by Advanced Life Sciences Inc. for further development.
On April 10, 2008, Advanced Life Sciences announced that, based on a productive meeting with the U.S. Food and Drug Administration (FDA), it plans to submit a New Drug Application (NDA) in the third quarter of 2008 for cethromycin to treat mild-to-moderate community acquired pneumonia.[2]
References
Categories:
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Macrolide antibiotics
- Quinolines
- Drug