Ceftibuten: Difference between revisions
No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
{{ | |authorTag={{GP}} | ||
{{ | |genericName=Ceftibuten | ||
== | |aOrAn=a | ||
|drugClass=3rd generation [[cephalosporin]] | |||
= | |indicationType=treatment | ||
[[ | |indication=acute bacterial exacerbations of [[chronic bronchitis]], acute bacterial [[otitis media]], [[pharyngitis]] and [[tonsillitis]] | ||
|adverseReactions=[[diarrhea]], [[nausea]], [[vomiting]] and [[headache]] | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult=Ceftibuten is indicated for the treatment of individuals with mild-to-moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. | |||
== | =====Acute Bacterial Exacerbations of Chronic Bronchitis===== | ||
* Due to [[Haemophilus influenzae]] (including β-lactamase-producing strains), Moraxella catarrhalis (including β-lactamase-producing strains), or [[Streptococcus pneumoniae]] (penicillin-susceptible strains only). | |||
* Dosage: 400 mg ORALLY once daily for 10 days | |||
=====Acute Bacterial Otitis Media===== | |||
* Due to [[Haemophilus influenzae]] (including β-lactamase-producing strains), [[Moraxella catarrhalis]] (including β-lactamase-producing strains), or [[Streptococcus pyogenes]]. | |||
* Dosage: 400 mg ORALLY once daily for 10 days | |||
== | =====Pharyngitis and Tonsillitis===== | ||
* Due to [[Streptococcus pyogenes]]. | |||
* Dosage: 400 mg ORALLY once daily for 10 days | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Ceftibuten in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Ceftibuten in adult patients. | |||
|fdaLIADPed=The safety and efficacy of ceftibuten in infants less than 6 months of age has not been established. | |||
Ceftibuten is indicated for the treatment of individuals with mild-to-moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below. | |||
=====Acute Bacterial Exacerbations of Chronic Bronchitis===== | |||
* Due to [[Haemophilus influenzae]] (including β-lactamase-producing strains), [[Moraxella catarrhalis]] (including β-lactamase-producing strains), or [[Streptococcus pneumoniae]] (penicillin-susceptible strains only). | |||
'''| [[Ceftibuten | * Dosage: 400 mg ORALLY once daily for 10 days | ||
=====Acute Bacterial Otitis Media===== | |||
* Due to [[Haemophilus influenzae]] (including β-lactamase-producing strains), [[Moraxella catarrhalis]] (including β-lactamase-producing strains), or [[Streptococcus pyogenes]]. | |||
== | * Dosage for 6 months to 12 years old: 9 mg/kg ORALLY once daily for 10 days; MAX, 400 mg/day | ||
* Dosage for 12 years and older: 400 mg ORALLY once daily for 10 days | |||
[[ | =====Pharyngitis and Tonsillitis===== | ||
[[ | * Due to [[Streptococcus pyogenes]]. | ||
* Dosage for 6 months to 12 years old: 9 mg/kg ORALLY once daily for 10 days; MAX, 400 mg/day | |||
* Dosage for 12 years and older: 400 mg ORALLY once daily for 10 days | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Ceftibuten in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Ceftibuten in pediatric patients. | |||
|contraindications=Ceftibuten is contraindicated in patients with known allergy to the cephalosporin group of antibiotics. | |||
|warnings=BEFORE THERAPY WITH CEFTIBUTEN IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFTIBUTEN, OTHER [[CEPHALOSPORINS]], PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO THE CEFTIBUTEN OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS [[ANTIHISTAMINES]], [[CORTICOSTEROIDS]], PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED. | |||
[[Pseudomembranous colitis]] has been reported with nearly all antibacterial agents, including ceftibuten, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with [[diarrhea]] subsequent to the administration of antibacterial agents. | |||
Treatment with antibacterial agents alters normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by [[Clostridium difficile]] is one primary cause of "antibiotic-associated colitis". | |||
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against [[Clostridium difficile]]. | |||
|clinicalTrials=======Ceftibuten Capsules (adult patients)====== | |||
In clinical trials, 1728 adult patients (1092 US and 636 international) were treated with the recommended dose of ceftibuten capsules (400 mg per day). There were no deaths or permanent disabilities thought due to drug toxicity in any of the patients in these studies. Thirty-six of 1728 (2%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. The discontinuations were primarily for gastrointestinal disturbances, usually diarrhea, vomiting, or nausea. Six of 1728 (0.3%) patients were discontinued due to [[rash]] or [[pruritus]] thought related to ceftibuten administration. | |||
In the US trials, the following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to ceftibuten capsules in multipledose clinical trials (n = 1092 ceftibuten-treated patients). | |||
[[File:Ceftibuten adverse reactions adult patients.png|thumb|none|600px]] | |||
======Ceftibuten oral suspension (pediatric patients)====== | |||
In clinical trials, 1152 pediatric patients (772 US and 380 international), 97% of whom were younger than 12 years of age, were treated with the recommended dose of ceftibuten (9 mg/kg once daily up to a maximum dose of 400 mg per day) for 10 days. There were no deaths, life-threatening adverse events, or permanent disabilities in any of the patients in these studies. Eight of 1152 (<1%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. The discontinuations were primarily (7 out of 8) for gastrointestinal disturbances, usually [[diarrhea]] or [[vomiting]]. One patient was discontinued due to a cutaneous rash thought possibly related to ceftibuten administration. | |||
In the US trials, the following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to ceftibuten oral suspension in multipledose clinical trials (n = 772 ceftibuten-treated patients). | |||
[[File:Ceftibuten adverse reactions pediatric patients.png|thumb|none|600px]] | |||
|postmarketing=The following adverse experiences have been reported during worldwide post-marketing surveillance: [[aphasia]], [[jaundice]], [[melena]], [[psychosis]], serum sickness-like reactions, [[stridor]], [[Stevens-Johnson syndrome]], and [[toxic epidermal necrolysis]]. | |||
======Cephalosporin-class Adverse Reactions====== | |||
In addition to the adverse reactions listed above that have been observed in patients treated with ceftibuten capsules, the following adverse events and altered laboratory tests have been reported for cephalosporin-class antibiotics: | |||
* [[allergic reactions]], [[anaphylaxis]], [[drug fever]], [[Stevens-Johnson syndrome]], [[renal dysfunction]], [[toxic nephropathy]], [[hepatic cholestasis]], [[aplastic anemia]], [[hemolytic anemia]], [[hemorrhage]], false-positive test for urinary glucose, [[neutropenia]], [[pancytopenia]], and [[agranulocytosis]]. [[Pseudomembranous colitis]]; onset of symptoms may occur during or after antibiotic treatment. | |||
Several cephalosporins have been implicated in triggering [[seizures]], particularly in patients with renal impairment when the dosage was not reduced. If [[seizures]] associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated. | |||
|drugInteractions======Theophylline===== | |||
Twelve healthy male volunteers were administered one 200-mg ceftibuten capsule twice daily for 6 days. With the morning dose of ceftibuten on day 6, each volunteer received a single intravenous infusion of theophylline (4 mg/kg). The pharmacokinetics of theophylline were not altered. The effect of ceftibuten on the pharmacokinetics of theophylline administered orally has not been investigated. | |||
=====Antacids or H2-receptor antagonists===== | |||
The effect of increased gastric pH on the bioavailability of ceftibuten was evaluated in 18 healthy adult volunteers. Each volunteer was administered one 400-mg ceftibuten capsule. A single dose of liquid antacid did not affect the Cmax or AUC of ceftibuten; however, 150 mg of ranitidine q12h for 3 days increased the ceftibuten Cmax by 23% and ceftibuten AUC by 16%. The clinical relevance of these increases is not known. | |||
=====Drug/Laboratory Test Interactions===== | |||
There have been no chemical or laboratory test interactions with ceftibuten noted to date. False-positive direct Coombs' tests have been reported during treatment with other cephalosporins. Therefore, it should be recognized that a positive Coombs' test could be due to the drug. The results of assays using red cells from healthy subjects to determine whether ceftibuten would cause direct Coombs' reactions in vitro showed no positive reaction at ceftibuten concentrations as high as 40 µg/mL. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=Ceftibuten was not teratogenic in the pregnant rat at oral doses up to 400 mg/kg/day (approximately 8.6 times the human dose based on mg/m2/day). Ceftibuten was not teratogenic in the pregnant rabbit at oral doses up to 40 mg/kg/day (approximately 1.5 times the human dose based on mg/m2/day) and has revealed no evidence of harm to the fetus. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |||
|useInLaborDelivery=Ceftibuten has not been studied for use during labor and delivery. Its use during such clinical situations should be weighed in terms of potential risk and benefit to both mother and fetus. | |||
|useInNursing=It is not known whether ceftibuten (at recommended dosages) is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ceftibuten is administered to a nursing woman. | |||
|useInPed=The safety and efficacy of ceftibuten in infants less than 6 months of age has not been established. | |||
|useInGeri=The usual adult dosage recommendation may be followed for patients in this age group. However, these patients should be monitored closely, particularly their renal function, as dosage adjustment may be required. | |||
|useInRenalImpair=Ceftibuten pharmacokinetics have been investigated in adult patients with renal dysfunction. The ceftibuten plasma half-life increased and apparent total clearance (CI/F) decreased proportionately with increasing degree of renal dysfunction. In 6 patients with moderate renal dysfunction (creatinine clearance 30 to 49 mL/min), the plasma half-life of ceftibuten increased to 7.1 hours and CI/F decreased to 30 mL/min. In 6 patients with severe renal dysfunction (creatinine clearance 5 to 29 mL/min), the half-life increased to 13.4 hours and CI/F decreased to 16 mL/min. In 6 functionally anephric patients (creatinine clearance <5 mL/min), the half-life increased to 22.3 hours and CI/F decreased to 11 mL/min (a 7- to 8-fold change compared to healthy volunteers). Hemodialysis removed 65% of the drug from the blood in 2 to 4 hours. These changes serve as the basis for dosage adjustment recommendations in adult patients with mild to severe renal dysfunction. | |||
|useInReproPotential=No impairment of fertility occurred when rats were administered ceftibuten orally up to 2000 mg/kg/day (approximately 43 times the human dose based on mg/m2/day). | |||
|administration=Oral | |||
|overdose=Overdosage of cephalosporins can cause cerebral irritation leading to convulsions. Ceftibuten is readily dialyzable and significant quantities (65% of plasma concentrations) can be removed from the circulation by a single hemodialysis session. Information does not exist with regard to removal of ceftibuten by peritoneal dialysis. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 443510665 | |||
| IUPAC_name = (6''R'',7''R'')-7-([(''Z'')-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino) -8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |||
| image = Ceftibuten structure.png | |||
| width = 300 | |||
<!--Clinical data--> | |||
| tradename = Cedax | |||
| Drugs.com = {{drugs.com|monograph|ceftibuten}} | |||
| MedlinePlus = a698023 | |||
| pregnancy_category = | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| metabolism = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 97519-39-6 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = DD14 | |||
| PubChem = 5282242 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB01415 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4445419 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = IW71N46B4Y | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00922 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 3510 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 1605 | |||
<!--Chemical data--> | |||
| C=15 | H=14 | N=4 | O=6 | S=2 | |||
| molecular_weight = 410.427 g.mol<sup>-1</sup> | |||
| smiles = O=C2N1/C(=C\CS[C@@H]1[C@@H]2NC(=O)C(=C/CC(=O)O)\c3nc(sc3)N)C(=O)O | |||
| InChI = 1/C15H14N4O6S2/c16-15-17-7(5-27-15)6(1-2-9(20)21)11(22)18-10-12(23)19-8(14(24)25)3-4-26-13(10)19/h1,3,5,10,13H,2,4H2,(H2,16,17)(H,18,22)(H,20,21)(H,24,25)/b6-1-/t10-,13-/m1/s1 | |||
| InChIKey = UNJFKXSSGBWRBZ-BJCIPQKHBU | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C15H14N4O6S2/c16-15-17-7(5-27-15)6(1-2-9(20)21)11(22)18-10-12(23)19-8(14(24)25)3-4-26-13(10)19/h1,3,5,10,13H,2,4H2,(H2,16,17)(H,18,22)(H,20,21)(H,24,25)/b6-1-/t10-,13-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = UNJFKXSSGBWRBZ-BJCIPQKHSA-N | |||
}} | |||
|mechAction=Ceftibuten exerts its bactericidal action by binding to essential target proteins of the bacterial cell wall. This binding leads to inhibition of cell-wall synthesis. | |||
Ceftibuten is stable in the presence of most plasmid-mediated beta-lactamases, but it is not stable in the presence of chromosomally-mediated cephalosporinases produced in organisms such as Bacteroides, Citrobacter, Enterobacter, Morganella, and Serratia. Like other beta-lactam agents, ceftibuten should not be used against strains resistant to beta-lactams due to general mechanisms such as permeability or penicillin-binding protein changes like penicillin-resistant S. pneumoniae. | |||
Ceftibuten has been shown to be active against most strains of the following organisms both in vitro and in clinical infections: | |||
* Gram-positive aerobes: | |||
:* Streptococcus pneumoniae (penicillin-susceptible strains only) Streptococcus pyogenes | |||
* Gram-negative aerobes: | |||
:* Haemophilus influenzae (including β-lactamase-producing strains) Moraxella catarrhalis (including β-lactamase-producing strains) | |||
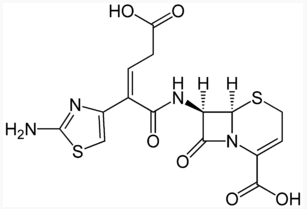
|structure=Ceftibuten dihydrate has the following structural formula: | |||
[[|File:Ceftibuten chemical structure.pngthumb|none|500px]] | |||
|PK======Absorption===== | |||
======Ceftibuten capsules====== | |||
Ceftibuten is rapidly absorbed after oral administration of Ceftibuten Capsules. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 400-mg dose of Ceftibuten Capsules to 12 healthy adult male volunteers (20 to 39 years of age) are displayed in the table below. When Ceftibuten Capsules were administered once daily for 7 days, the average Cmax was 17.9 µg/mL on day 7. Therefore, ceftibuten accumulation in plasma is about 20% at steady state. | |||
======Ceftibuten oral suspension====== | |||
Ceftibuten is rapidly absorbed after oral administration of Ceftibuten Oral Suspension. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 9-mg/kg dose of Ceftibuten Oral Suspension to 32 fasting pediatric patients (6 months to 12 years of age) are displayed in the following table: | |||
[[File:Ceftibuten pharmacokinetics.png|thumb|none|600px]] | |||
The absolute bioavailability of Ceftibuten Oral Suspension has not been determined. The plasma concentrations of ceftibuten in pediatric patients are dose proportional following single doses of Ceftibuten Capsules of 200 mg and 400 mg and of Ceftibuten Oral Suspension between 4.5 mg/kg and 9 mg/kg. | |||
=====Distribution===== | |||
=====Ceftibuten capsules====== | |||
The average apparent volume of distribution (V/F) of ceftibuten in 6 adult subjects is 0.21 L/kg (± 1 SD = 0.03 L/kg). | |||
=====Ceftabuten oral suspension===== | |||
The average apparent volume of distribution (V/F) of ceftibuten in 32 fasting pediatric patients is 0.5 L/kg (± 1 SD = 0.2 L/kg). | |||
=====Protein Binding===== | |||
Ceftibuten is 65% bound to plasma proteins. The protein binding is independent of plasma ceftibuten concentration. | |||
=====Tissue Penetration===== | |||
======Bronchial secretions====== | |||
In a study of 15 adults administered a single 400-mg dose of ceftibuten and scheduled to undergo bronchoscopy, the mean concentrations in epithelial lining fluid and bronchial mucosa were 15% and 37%, respectively, of the plasma concentrations. | |||
======Sputum====== | |||
Ceftibuten sputum levels average approximately 7% of the concomitant plasma ceftibuten level. In a study of 24 adults administered ceftibuten 200 mg bid or 400 mg qd, the average Cmax in sputum (1.5 µg/mL) occurred at 2 hours postdose and the average Cmax in plasma (17 µg/mL) occurred at 2 hours postdose. | |||
======Middle-ear fluid (MEF)====== | |||
In a study of 12 pediatric patients administered 9 mg/kg, ceftibuten MEF area under the curve (AUC) averaged approximately 70% of the plasma AUC. In the same study, Cmax values were 14.3 ± 2.7 µg/mL in MEF at 4 hours postdose and 14.5 ± 3.7 µg/mL in plasma at 2 hours postdose. | |||
======Tonsillar tissue====== | |||
Data on ceftibuten penetration into tonsillar tissue are not available. | |||
======Cerebrospinal fluid====== | |||
Data on ceftibuten penetration into cerebrospinal fluid are not available. | |||
=====Metabolism and Excretion===== | |||
A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer. The trans-isomer is approximately ⅛ as antimicrobially potent as the cis-isomer. | |||
Ceftibuten is excreted in the urine; 95% of the administered radioactivity was recovered either in urine or feces. In 6 healthy adult male volunteers, approximately 56% of the administered dose of ceftibuten was recovered from urine and 39% from the feces within 24 hours. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment. | |||
=====Food Effect on Absorption===== | |||
Food affects the bioavailability of ceftibuten from Cefitbuten Capsules and Ceftibuten Oral Suspension. | |||
The effect of food on the bioavailability of Ceftibuten Capsules was evaluated in 26 healthy adult male volunteers who ingested 400 mg of Ceftibuten Capsules after an overnight fast or immediately after a standardized breakfast. Results showed that food delays the time of Cmax by 1.75 hours, decreases the Cmax by 18%, and decreases the extent of absorption (AUC) by 8%. | |||
The effect of food on the bioavailability of Ceftibuten Oral Suspension was evaluated in 18 healthy adult male volunteers who ingested 400 mg of Ceftibuten Oral Suspension after an overnight fast or immediately after a standardized breakfast. Results obtained demonstrated a decrease in Cmax of 26% and an AUC of 17% when Ceftibuten Oral Suspension was administered with a high-fat breakfast, and a decrease in Cmax of 17% and an AUC of 12% when Ceftibuten Oral Suspension was administered with a low-calorie nonfat breakfast. | |||
|nonClinToxic=======Carcinogenesis and Mutagenesis====== | |||
Long-term animal studies have not been performed to evaluate the carcinogenic potential of ceftibuten. No mutagenic effects were seen in the following studies: in vitro chromosome assay in human lymphocytes, in vivo chromosome assay in mouse bone marrow cells, Chinese Hamster Ovary (CHO) cell point mutation assay at the hypoxanthine-guanine phosphoribosyl transferase (HGPRT) locus, and in a bacterial reversion point mutation test (Ames). | |||
|clinicalStudies======Acute Bacterial Exacerbations of Chronic Bronchitis===== | |||
Three clinical trials (two domestic, the third abroad) have been conducted testing ceftibuten in the treatment of acute exacerbations of chronic bronchitis (AECB). Overall, the clinical outcome among patients who had signs and symptoms of AECB, who had a gram stain showing a predominance of PMNs and few epithelial cells, and who were evaluated at approximately 1 to 2 weeks after completing therapy were equivalent to comparators. The bacterial eradication rates of specific pathogens are presented below. | |||
[[File:Ceftibuten clinical studies Acute Bacterial Exacerbations of Chronic Bronchitis.png|thumb|none|500px]] | |||
=====Acute Bacterial Otitis Media===== | |||
Four clinical trials (three domestic, the fourth abroad) have been conducted testing ceftibuten in the treatment of acute bacterial otitis media. Overall, the clinical outcome among patients who had signs and symptoms of acute bacterial otitis media and who were evaluated at approximately 1 to 2 weeks after completing therapy were equivalent to comparators. Tympanocentesis was performed on patients in three of the above-mentioned studies; the bacterial eradication rates of specific pathogens are presented below. | |||
[[File:Ceftibuten clinical studies Acute Bacterial Otitis Media.png|thumb|none|500px]] | |||
|howSupplied=* Ceftibuten Capsules, 400 mg | |||
:* 20 Capsules/Bottle (NDC 44183-400-22) | |||
* Ceftibuten oral suspension, 180 mg/5 mL | |||
:* 36 mg/mL 30-mL Bottle (NDC 44183-180-30) | |||
:* 36 mg/mL 60-mL Bottle (NDC 44183-180-02) | |||
|storage=* Store the capsules between 2° and 25°C (36° and 77°F). | |||
* Store de oral suspension between 2° and 25°C (36° and 77°F). | |||
|packLabel=[[File:Ceftibuten capsules FDA package label.png|thumb|none|600px]] | |||
[[File:Ceftibuten oral suspension FDA package label.png|thumb|none|600px]] | |||
|fdaPatientInfo=Patients should be informed that: | |||
* If the patient is diabetic, he/she should be informed that Ceftibuten Oral Suspension contains 1 gram sucrose per teaspoon of suspension. | |||
* Ceftibuten Oral Suspension should be taken at least 2 hours before a meal or at least 1 hour after a meal | |||
|alcohol=Alcohol-Ceftibuten interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=* Cedax <ref>{{cite web|url=http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=13229b1a-c1db-4303-8e73-f83fa1e5677c|title=FDA LABEL: CEFTIBUTEN- ceftibuten dihydrate capsule ceftibuten dihydrate suspension}}</ref> | |||
}} | |||
{{LabelImage | |||
|fileName=Ceftibuten capsules 400 mg.png | |||
}} | |||
{{LabelImage | |||
|fileName=Ceftibuten oral suspension 180 mg per 5 ml.png | |||
}} | |||
Latest revision as of 17:53, 12 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ceftibuten is a 3rd generation cephalosporin that is FDA approved for the treatment of acute bacterial exacerbations of chronic bronchitis, acute bacterial otitis media, pharyngitis and tonsillitis. Common adverse reactions include diarrhea, nausea, vomiting and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Ceftibuten is indicated for the treatment of individuals with mild-to-moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below.
Acute Bacterial Exacerbations of Chronic Bronchitis
- Due to Haemophilus influenzae (including β-lactamase-producing strains), Moraxella catarrhalis (including β-lactamase-producing strains), or Streptococcus pneumoniae (penicillin-susceptible strains only).
- Dosage: 400 mg ORALLY once daily for 10 days
Acute Bacterial Otitis Media
- Due to Haemophilus influenzae (including β-lactamase-producing strains), Moraxella catarrhalis (including β-lactamase-producing strains), or Streptococcus pyogenes.
- Dosage: 400 mg ORALLY once daily for 10 days
Pharyngitis and Tonsillitis
- Due to Streptococcus pyogenes.
- Dosage: 400 mg ORALLY once daily for 10 days
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftibuten in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceftibuten in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and efficacy of ceftibuten in infants less than 6 months of age has not been established. Ceftibuten is indicated for the treatment of individuals with mild-to-moderate infections caused by susceptible strains of the designated microorganisms in the specific conditions listed below.
Acute Bacterial Exacerbations of Chronic Bronchitis
- Due to Haemophilus influenzae (including β-lactamase-producing strains), Moraxella catarrhalis (including β-lactamase-producing strains), or Streptococcus pneumoniae (penicillin-susceptible strains only).
- Dosage: 400 mg ORALLY once daily for 10 days
Acute Bacterial Otitis Media
- Due to Haemophilus influenzae (including β-lactamase-producing strains), Moraxella catarrhalis (including β-lactamase-producing strains), or Streptococcus pyogenes.
- Dosage for 6 months to 12 years old: 9 mg/kg ORALLY once daily for 10 days; MAX, 400 mg/day
- Dosage for 12 years and older: 400 mg ORALLY once daily for 10 days
Pharyngitis and Tonsillitis
- Due to Streptococcus pyogenes.
- Dosage for 6 months to 12 years old: 9 mg/kg ORALLY once daily for 10 days; MAX, 400 mg/day
- Dosage for 12 years and older: 400 mg ORALLY once daily for 10 days
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftibuten in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceftibuten in pediatric patients.
Contraindications
Ceftibuten is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
Warnings
BEFORE THERAPY WITH CEFTIBUTEN IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFTIBUTEN, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO THE CEFTIBUTEN OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including ceftibuten, and may range in severity from mild to life threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
Treatment with antibacterial agents alters normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is one primary cause of "antibiotic-associated colitis".
After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile.
Adverse Reactions
Clinical Trials Experience
Ceftibuten Capsules (adult patients)
In clinical trials, 1728 adult patients (1092 US and 636 international) were treated with the recommended dose of ceftibuten capsules (400 mg per day). There were no deaths or permanent disabilities thought due to drug toxicity in any of the patients in these studies. Thirty-six of 1728 (2%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. The discontinuations were primarily for gastrointestinal disturbances, usually diarrhea, vomiting, or nausea. Six of 1728 (0.3%) patients were discontinued due to rash or pruritus thought related to ceftibuten administration.
In the US trials, the following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to ceftibuten capsules in multipledose clinical trials (n = 1092 ceftibuten-treated patients).
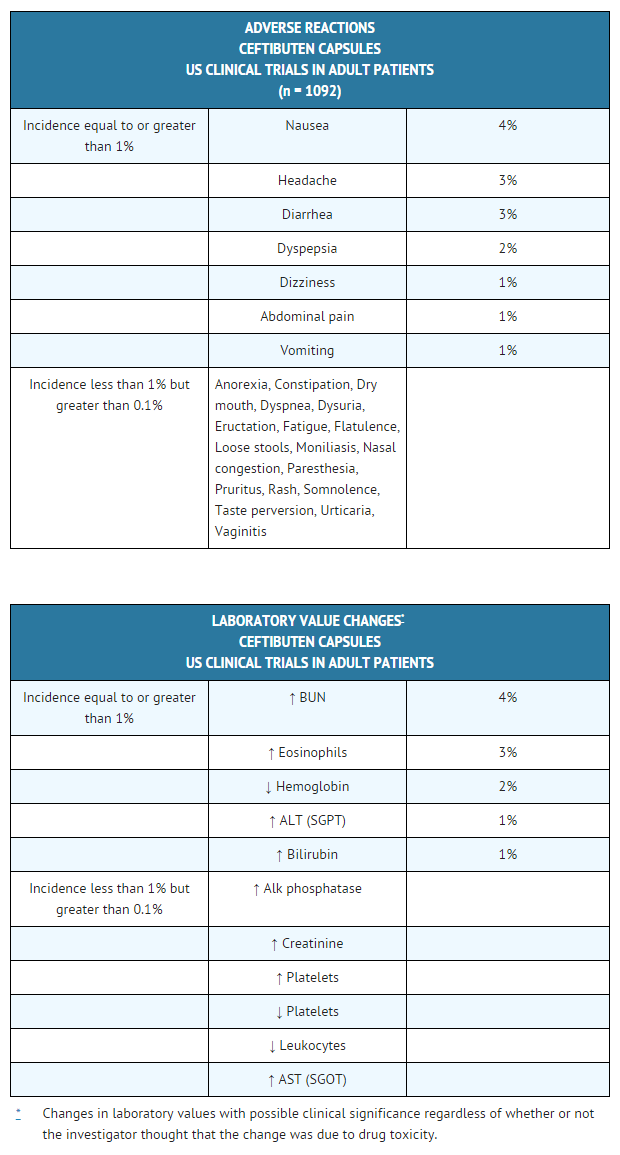
Ceftibuten oral suspension (pediatric patients)
In clinical trials, 1152 pediatric patients (772 US and 380 international), 97% of whom were younger than 12 years of age, were treated with the recommended dose of ceftibuten (9 mg/kg once daily up to a maximum dose of 400 mg per day) for 10 days. There were no deaths, life-threatening adverse events, or permanent disabilities in any of the patients in these studies. Eight of 1152 (<1%) patients discontinued medication due to adverse events thought by the investigators to be possibly, probably, or almost certainly related to drug toxicity. The discontinuations were primarily (7 out of 8) for gastrointestinal disturbances, usually diarrhea or vomiting. One patient was discontinued due to a cutaneous rash thought possibly related to ceftibuten administration.
In the US trials, the following adverse events were thought by the investigators to be possibly, probably, or almost certainly related to ceftibuten oral suspension in multipledose clinical trials (n = 772 ceftibuten-treated patients).
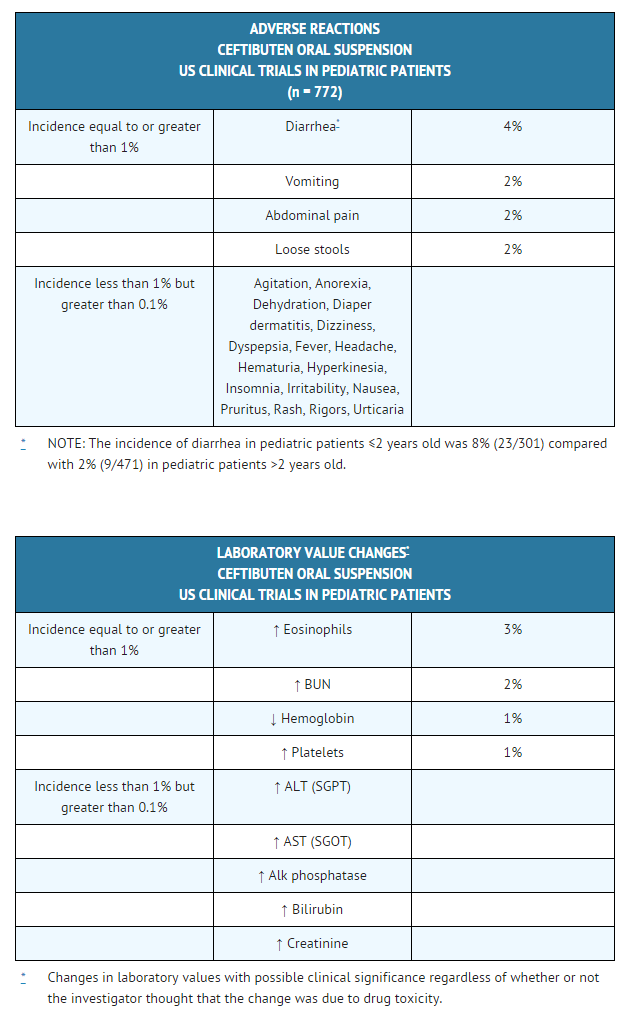
Postmarketing Experience
The following adverse experiences have been reported during worldwide post-marketing surveillance: aphasia, jaundice, melena, psychosis, serum sickness-like reactions, stridor, Stevens-Johnson syndrome, and toxic epidermal necrolysis.
Cephalosporin-class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with ceftibuten capsules, the following adverse events and altered laboratory tests have been reported for cephalosporin-class antibiotics:
- allergic reactions, anaphylaxis, drug fever, Stevens-Johnson syndrome, renal dysfunction, toxic nephropathy, hepatic cholestasis, aplastic anemia, hemolytic anemia, hemorrhage, false-positive test for urinary glucose, neutropenia, pancytopenia, and agranulocytosis. Pseudomembranous colitis; onset of symptoms may occur during or after antibiotic treatment.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Drug Interactions
Theophylline
Twelve healthy male volunteers were administered one 200-mg ceftibuten capsule twice daily for 6 days. With the morning dose of ceftibuten on day 6, each volunteer received a single intravenous infusion of theophylline (4 mg/kg). The pharmacokinetics of theophylline were not altered. The effect of ceftibuten on the pharmacokinetics of theophylline administered orally has not been investigated.
Antacids or H2-receptor antagonists
The effect of increased gastric pH on the bioavailability of ceftibuten was evaluated in 18 healthy adult volunteers. Each volunteer was administered one 400-mg ceftibuten capsule. A single dose of liquid antacid did not affect the Cmax or AUC of ceftibuten; however, 150 mg of ranitidine q12h for 3 days increased the ceftibuten Cmax by 23% and ceftibuten AUC by 16%. The clinical relevance of these increases is not known.
Drug/Laboratory Test Interactions
There have been no chemical or laboratory test interactions with ceftibuten noted to date. False-positive direct Coombs' tests have been reported during treatment with other cephalosporins. Therefore, it should be recognized that a positive Coombs' test could be due to the drug. The results of assays using red cells from healthy subjects to determine whether ceftibuten would cause direct Coombs' reactions in vitro showed no positive reaction at ceftibuten concentrations as high as 40 µg/mL.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Ceftibuten was not teratogenic in the pregnant rat at oral doses up to 400 mg/kg/day (approximately 8.6 times the human dose based on mg/m2/day). Ceftibuten was not teratogenic in the pregnant rabbit at oral doses up to 40 mg/kg/day (approximately 1.5 times the human dose based on mg/m2/day) and has revealed no evidence of harm to the fetus. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ceftibuten in women who are pregnant.
Labor and Delivery
Ceftibuten has not been studied for use during labor and delivery. Its use during such clinical situations should be weighed in terms of potential risk and benefit to both mother and fetus.
Nursing Mothers
It is not known whether ceftibuten (at recommended dosages) is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ceftibuten is administered to a nursing woman.
Pediatric Use
The safety and efficacy of ceftibuten in infants less than 6 months of age has not been established.
Geriatic Use
The usual adult dosage recommendation may be followed for patients in this age group. However, these patients should be monitored closely, particularly their renal function, as dosage adjustment may be required.
Gender
There is no FDA guidance on the use of Ceftibuten with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ceftibuten with respect to specific racial populations.
Renal Impairment
Ceftibuten pharmacokinetics have been investigated in adult patients with renal dysfunction. The ceftibuten plasma half-life increased and apparent total clearance (CI/F) decreased proportionately with increasing degree of renal dysfunction. In 6 patients with moderate renal dysfunction (creatinine clearance 30 to 49 mL/min), the plasma half-life of ceftibuten increased to 7.1 hours and CI/F decreased to 30 mL/min. In 6 patients with severe renal dysfunction (creatinine clearance 5 to 29 mL/min), the half-life increased to 13.4 hours and CI/F decreased to 16 mL/min. In 6 functionally anephric patients (creatinine clearance <5 mL/min), the half-life increased to 22.3 hours and CI/F decreased to 11 mL/min (a 7- to 8-fold change compared to healthy volunteers). Hemodialysis removed 65% of the drug from the blood in 2 to 4 hours. These changes serve as the basis for dosage adjustment recommendations in adult patients with mild to severe renal dysfunction.
Hepatic Impairment
There is no FDA guidance on the use of Ceftibuten in patients with hepatic impairment.
Females of Reproductive Potential and Males
No impairment of fertility occurred when rats were administered ceftibuten orally up to 2000 mg/kg/day (approximately 43 times the human dose based on mg/m2/day).
Immunocompromised Patients
There is no FDA guidance one the use of Ceftibuten in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information regarding Ceftibuten Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ceftibuten and IV administrations.
Overdosage
Overdosage of cephalosporins can cause cerebral irritation leading to convulsions. Ceftibuten is readily dialyzable and significant quantities (65% of plasma concentrations) can be removed from the circulation by a single hemodialysis session. Information does not exist with regard to removal of ceftibuten by peritoneal dialysis.
Pharmacology
| |
Ceftibuten
| |
| Systematic (IUPAC) name | |
| (6R,7R)-7-([(Z)-2-(2-amino-1,3-thiazol-4-yl)-5-hydroxy-5-oxopent-2-enoyl]amino) -8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | J01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 410.427 g.mol-1 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
Ceftibuten exerts its bactericidal action by binding to essential target proteins of the bacterial cell wall. This binding leads to inhibition of cell-wall synthesis.
Ceftibuten is stable in the presence of most plasmid-mediated beta-lactamases, but it is not stable in the presence of chromosomally-mediated cephalosporinases produced in organisms such as Bacteroides, Citrobacter, Enterobacter, Morganella, and Serratia. Like other beta-lactam agents, ceftibuten should not be used against strains resistant to beta-lactams due to general mechanisms such as permeability or penicillin-binding protein changes like penicillin-resistant S. pneumoniae.
Ceftibuten has been shown to be active against most strains of the following organisms both in vitro and in clinical infections:
- Gram-positive aerobes:
- Streptococcus pneumoniae (penicillin-susceptible strains only) Streptococcus pyogenes
- Gram-negative aerobes:
- Haemophilus influenzae (including β-lactamase-producing strains) Moraxella catarrhalis (including β-lactamase-producing strains)
Structure
Ceftibuten dihydrate has the following structural formula:
[[|File:Ceftibuten chemical structure.pngthumb|none|500px]]
Pharmacodynamics
There is limited information regarding Ceftibuten Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
Ceftibuten capsules
Ceftibuten is rapidly absorbed after oral administration of Ceftibuten Capsules. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 400-mg dose of Ceftibuten Capsules to 12 healthy adult male volunteers (20 to 39 years of age) are displayed in the table below. When Ceftibuten Capsules were administered once daily for 7 days, the average Cmax was 17.9 µg/mL on day 7. Therefore, ceftibuten accumulation in plasma is about 20% at steady state.
Ceftibuten oral suspension
Ceftibuten is rapidly absorbed after oral administration of Ceftibuten Oral Suspension. The plasma concentrations and pharmacokinetic parameters of ceftibuten after a single 9-mg/kg dose of Ceftibuten Oral Suspension to 32 fasting pediatric patients (6 months to 12 years of age) are displayed in the following table:
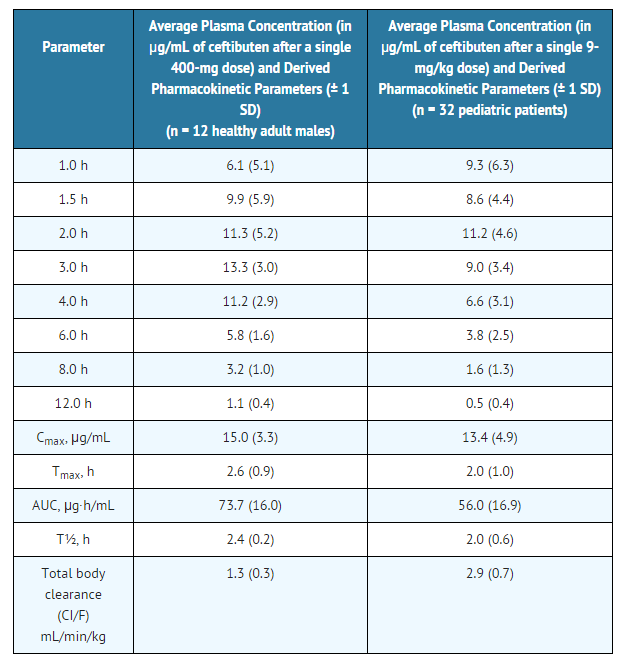
The absolute bioavailability of Ceftibuten Oral Suspension has not been determined. The plasma concentrations of ceftibuten in pediatric patients are dose proportional following single doses of Ceftibuten Capsules of 200 mg and 400 mg and of Ceftibuten Oral Suspension between 4.5 mg/kg and 9 mg/kg.
Distribution
Ceftibuten capsules=
The average apparent volume of distribution (V/F) of ceftibuten in 6 adult subjects is 0.21 L/kg (± 1 SD = 0.03 L/kg).
Ceftabuten oral suspension
The average apparent volume of distribution (V/F) of ceftibuten in 32 fasting pediatric patients is 0.5 L/kg (± 1 SD = 0.2 L/kg).
Protein Binding
Ceftibuten is 65% bound to plasma proteins. The protein binding is independent of plasma ceftibuten concentration.
Tissue Penetration
Bronchial secretions
In a study of 15 adults administered a single 400-mg dose of ceftibuten and scheduled to undergo bronchoscopy, the mean concentrations in epithelial lining fluid and bronchial mucosa were 15% and 37%, respectively, of the plasma concentrations.
Sputum
Ceftibuten sputum levels average approximately 7% of the concomitant plasma ceftibuten level. In a study of 24 adults administered ceftibuten 200 mg bid or 400 mg qd, the average Cmax in sputum (1.5 µg/mL) occurred at 2 hours postdose and the average Cmax in plasma (17 µg/mL) occurred at 2 hours postdose.
Middle-ear fluid (MEF)
In a study of 12 pediatric patients administered 9 mg/kg, ceftibuten MEF area under the curve (AUC) averaged approximately 70% of the plasma AUC. In the same study, Cmax values were 14.3 ± 2.7 µg/mL in MEF at 4 hours postdose and 14.5 ± 3.7 µg/mL in plasma at 2 hours postdose.
Tonsillar tissue
Data on ceftibuten penetration into tonsillar tissue are not available.
Cerebrospinal fluid
Data on ceftibuten penetration into cerebrospinal fluid are not available.
Metabolism and Excretion
A study with radiolabeled ceftibuten administered to 6 healthy adult male volunteers demonstrated that cis-ceftibuten is the predominant component in both plasma and urine. About 10% of ceftibuten is converted to the trans-isomer. The trans-isomer is approximately ⅛ as antimicrobially potent as the cis-isomer.
Ceftibuten is excreted in the urine; 95% of the administered radioactivity was recovered either in urine or feces. In 6 healthy adult male volunteers, approximately 56% of the administered dose of ceftibuten was recovered from urine and 39% from the feces within 24 hours. Because renal excretion is a significant pathway of elimination, patients with renal dysfunction and patients undergoing hemodialysis require dosage adjustment.
Food Effect on Absorption
Food affects the bioavailability of ceftibuten from Cefitbuten Capsules and Ceftibuten Oral Suspension.
The effect of food on the bioavailability of Ceftibuten Capsules was evaluated in 26 healthy adult male volunteers who ingested 400 mg of Ceftibuten Capsules after an overnight fast or immediately after a standardized breakfast. Results showed that food delays the time of Cmax by 1.75 hours, decreases the Cmax by 18%, and decreases the extent of absorption (AUC) by 8%.
The effect of food on the bioavailability of Ceftibuten Oral Suspension was evaluated in 18 healthy adult male volunteers who ingested 400 mg of Ceftibuten Oral Suspension after an overnight fast or immediately after a standardized breakfast. Results obtained demonstrated a decrease in Cmax of 26% and an AUC of 17% when Ceftibuten Oral Suspension was administered with a high-fat breakfast, and a decrease in Cmax of 17% and an AUC of 12% when Ceftibuten Oral Suspension was administered with a low-calorie nonfat breakfast.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
Long-term animal studies have not been performed to evaluate the carcinogenic potential of ceftibuten. No mutagenic effects were seen in the following studies: in vitro chromosome assay in human lymphocytes, in vivo chromosome assay in mouse bone marrow cells, Chinese Hamster Ovary (CHO) cell point mutation assay at the hypoxanthine-guanine phosphoribosyl transferase (HGPRT) locus, and in a bacterial reversion point mutation test (Ames).
Clinical Studies
Acute Bacterial Exacerbations of Chronic Bronchitis
Three clinical trials (two domestic, the third abroad) have been conducted testing ceftibuten in the treatment of acute exacerbations of chronic bronchitis (AECB). Overall, the clinical outcome among patients who had signs and symptoms of AECB, who had a gram stain showing a predominance of PMNs and few epithelial cells, and who were evaluated at approximately 1 to 2 weeks after completing therapy were equivalent to comparators. The bacterial eradication rates of specific pathogens are presented below.
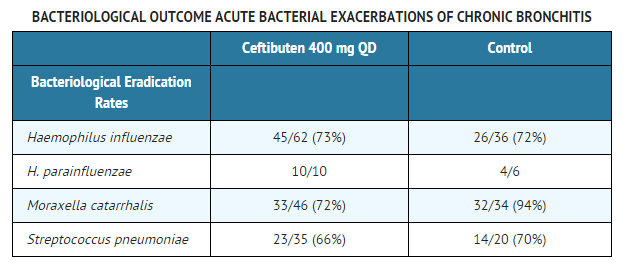
Acute Bacterial Otitis Media
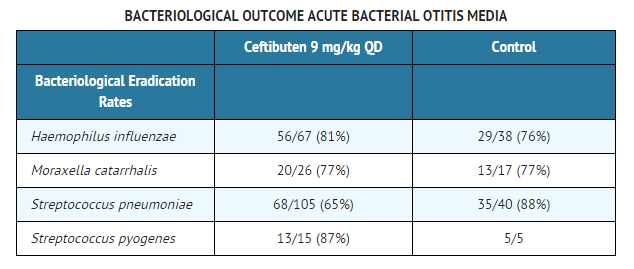
Four clinical trials (three domestic, the fourth abroad) have been conducted testing ceftibuten in the treatment of acute bacterial otitis media. Overall, the clinical outcome among patients who had signs and symptoms of acute bacterial otitis media and who were evaluated at approximately 1 to 2 weeks after completing therapy were equivalent to comparators. Tympanocentesis was performed on patients in three of the above-mentioned studies; the bacterial eradication rates of specific pathogens are presented below.
How Supplied
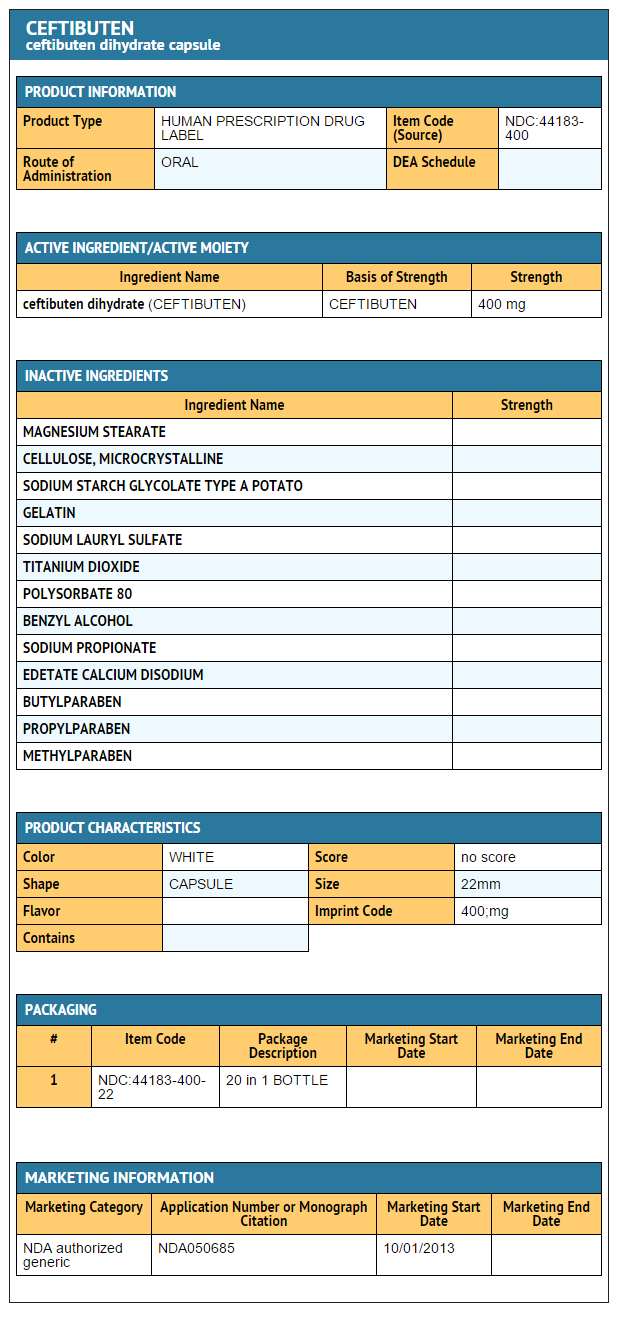
- Ceftibuten Capsules, 400 mg
- 20 Capsules/Bottle (NDC 44183-400-22)
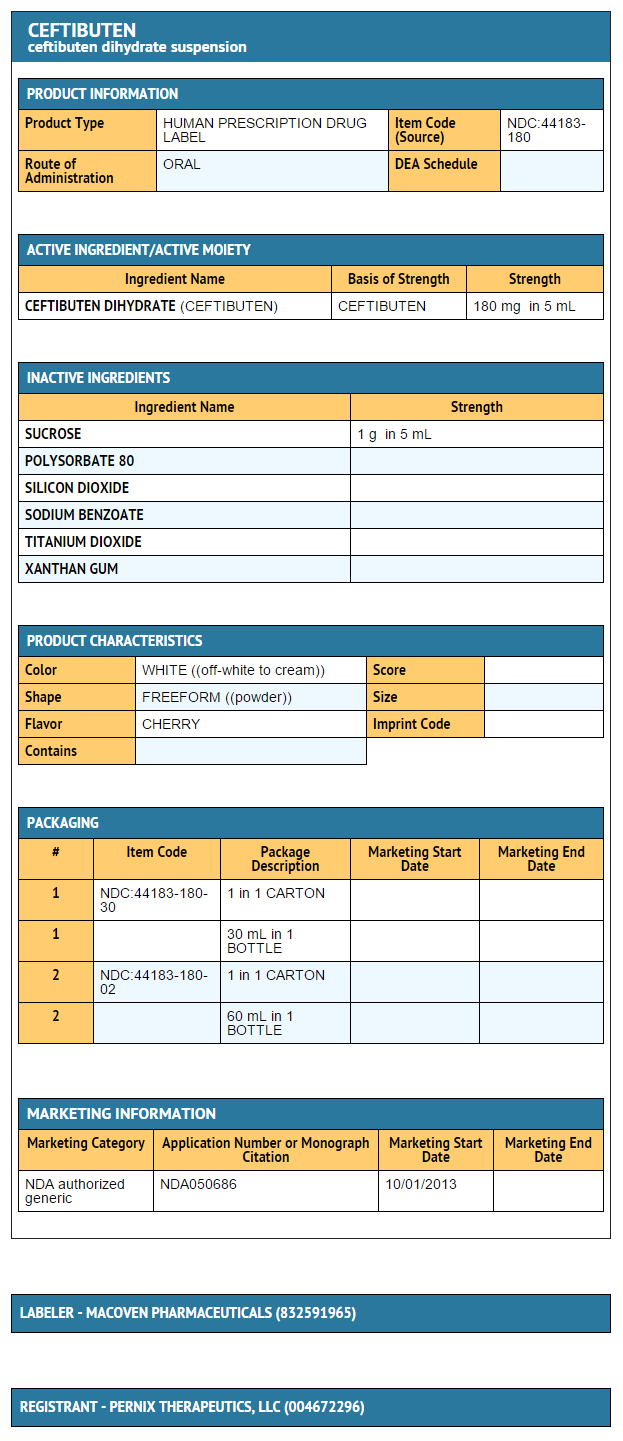
- Ceftibuten oral suspension, 180 mg/5 mL
- 36 mg/mL 30-mL Bottle (NDC 44183-180-30)
- 36 mg/mL 60-mL Bottle (NDC 44183-180-02)
Storage
- Store the capsules between 2° and 25°C (36° and 77°F).
- Store de oral suspension between 2° and 25°C (36° and 77°F).
Images
Drug Images
{{#ask: Page Name::Ceftibuten |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ceftibuten |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be informed that:
- If the patient is diabetic, he/she should be informed that Ceftibuten Oral Suspension contains 1 gram sucrose per teaspoon of suspension.
- Ceftibuten Oral Suspension should be taken at least 2 hours before a meal or at least 1 hour after a meal
Precautions with Alcohol
Alcohol-Ceftibuten interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Cedax [1]
Look-Alike Drug Names
There is limited information regarding Ceftibuten Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Ceftibuten |Label Name=Ceftibuten capsules 400 mg.png
}}
{{#subobject:
|Label Page=Ceftibuten |Label Name=Ceftibuten oral suspension 180 mg per 5 ml.png
}}