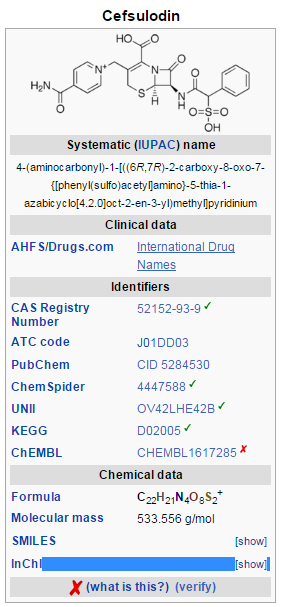
Cefsulodin
|
WikiDoc Resources for Cefsulodin |
|
Articles |
|---|
|
Most recent articles on Cefsulodin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Cefsulodin at Clinical Trials.gov Clinical Trials on Cefsulodin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Cefsulodin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Cefsulodin Discussion groups on Cefsulodin Patient Handouts on Cefsulodin Directions to Hospitals Treating Cefsulodin Risk calculators and risk factors for Cefsulodin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Cefsulodin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Overview
Cefsulodin is a third-generation cephalosporin antibiotic with specific activity against Pseudomonas aeruginosa. It has no significant activity against other Gram-negative bacteria and very limited activity against Gram-positive bacteria and anaerobic bacteria. Cefsulodin was first synthesized and patented by the Takeda Pharmaceutical Company in 1977. In 2002, Takeda stopped production of cefsulodin. Many years of low-stability cefsulodin production has led to a widespread reduction of laboratory and research uses. Current attempts (i.e. IDEXX Laboratories) of increasing purity and stability of cefsulodin center around recrystallization. Typically, the process entails: Cefsulodin is dissolved in an organic solvent, sodium ions, water, or any mixture thereof, then subsequently recrystallized through separation of the unwanted fraction. Recently, TOKU-E has found the main cause of cefsulodin instability stems from one key impurity in 7-aminocephalosporanic acid, a raw material used in the synthesis of cefsulodin. To produce high-purity, high-stability cefsulodin, TOKU-E uses industrial HPLC to remove significant quantities of this impurity in 7-ACA and thus produces ultrapure, ultrastable, and ultrapotent cefsulodin.[1]
General use
Cefuslodin is most commonly used in cefsulodin-irgasan-novobiocin agar to select for Yersinia microorganisms.[2] This agar is most often used in water and beverage testing.
Susceptibility data
The following represents MIC susceptibility data for various P. aeruginosa strains.
- Pseudomonas aeruginosa PA13 (resistant strain): 32 μg/ml
- Pseudomonas aeruginosa (wild-type, susceptible): 4 - 8 μg/ml
References
- ↑ [1], TOKU-E Technical Application Sheet.
- ↑ "BAM Media M35: Cefsulodin-Irgasan Novobiocin Agar or Yersinia Selective Agar". Retrieved 2 September 2012.
- ↑ http://antibiotics.toku-e.com/antimicrobial_474.html