Cefditoren
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cefditoren is a 3rd generation cephalosporin that is FDA approved for the treatment of acute bacterial exacerbation of chronic bronchitis, community acquired pneumonia, infection of skin and/or subcutaneous tissue, and pharyngitis/tonsillitis. Common adverse reactions include diarrhea, nausea and candida vaginitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute Bacterial Exacerbation of Chronic Bronchitis
- Caused by Haemophilus influenzae (including ß-lactamase-producing strains), Haemophilus parainfluenzae (including ß-lactamase producing strains), Streptococcus pneumoniae (penicillin susceptible strains only), or Moraxella catarrhalis (including ß-lactamase-producing strains).
Community-Acquired Pneumonia
- Caused by Haemophilus influenzae (including ß-lactamase-producing strains), Haemophilus parainfluenzae (including ß-lactamase-producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), or Moraxella catarrhalis (including ß-lactamase producing strains).
Pharyngitis/Tonsillitis
- Caused by Streptococcus pyogenes. NOTE: Cefditoren Pivoxil is effective in the eradication of Streptococcus pyogenes from the oropharynx. Cefditoren Pivoxil Tablets has not been studied for the prevention of rheumatic fever following Streptococcus pyogenes pharyngitis/tonsillitis. Only intramuscular penicillin has been demonstrated to be effective for the prevention of rheumatic fever.
Uncomplicated Skin and Skin-Structure Infections
- Caused by Staphylococcus aureus (including ß-lactamase-producing strains) or Streptococcus pyogenes.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefditoren in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cefditoren in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Use of cefditoren pivoxil is not recommended for pediatric patients less than 12 years of age. The safety and efficacy of cefditoren pivoxil tablets in this population, including any effects of altered carnitine concentration, have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cefditoren in pediatric patients.
Non–Guideline-Supported Use
Otitis media
- Cefditoren pivoxil 9 milligrams/kilogram (mg/kg) daily.
Contraindications
- Cefditoren Pivoxil is contraindicated in patients with known allergy to the cephalosporin class of antibiotics or any of its components.
- Cefditoren Pivoxil is contraindicated in patients with carnitine deficiency or inborn errors of metabolism that may result in clinically significant carnitine deficiency, because use of Cefditoren Pivoxil causes renal excretion of carnitine.
- Cefditoren Pivoxil tablets contain sodium caseinate, a milk protein. Patients with milk protein hypersensitivity (not lactose intolerance) should not be administered Cefditoren Pivoxil.
Warnings
- Before therapy with cefditoren pivoxil is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cefditoren pivoxil, other cephalosporins, penicillins, or other drugs. If cefditoren pivoxil is to be given to penicillin-sensitive patients, caution should be exercised because cross-hypersensitivity among ß-lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction to cefditoren pivoxil occurs, the drug should be discontinued. Serious acute hypersensitivity reactions may require treatment with epinephrine and other emergency measures, including oxygen, intravenous fluids, intravenous antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated.
- Pseudomembranous colitis has been reported with nearly all antibacterial agents, including cefditoren pivoxil, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of antibacterial agents.
- Treatment with antibacterial agents alters normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile (C. difficile) is a primary cause of antibiotic-associated colitis.
- After the diagnosis of pseudomembranous colitis has been established, appropriate therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis.
Precautions
- Prescribing Cefditoren Pivoxil in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
- Cefditoren Pivoxil is not recommended when prolonged antibiotic treatment is necessary, since other pivalate-containing compounds have caused clinical manifestations of carnitine deficiency when used over a period of months. No clinical effects of carnitine decrease have been associated with short-term treatment. The effects on carnitine concentrations of repeat short-term courses of Cefditoren Pivoxil are not known.
- In community-acquired pneumonia patients (N=192, mean age 50.3 ± 17.2 years) given a 200 mg BID regimen for 14 days, the mean decrease in serum concentrations of total carnitine while on therapy was 13.8 ± 10.8 nmole/mL, representing a 30% decrease in serum carnitine concentrations. In community-acquired pneumonia patients (N=192, mean age 51.3 ± 17.8 years) given a 400 mg BID regimen for 14 days, the mean decrease in serum concentrations of total carnitine while on therapy was 21.5 ± 13.1 mole/mL, representing a 46% decrease in serum carnitine concentrations. Plasma concentrations of carnitine returned to the normal control range within 7 days after discontinuation of cefditoren pivoxil. Comparable decreases in carnitine were observed in healthy volunteers (mean age 33.6 ± 7.4 years) following a 200 mg or 400 mg BID regimen. Community-acquired pneumonia clinical trials demonstrated no adverse events attributable to decreases in serum carnitine concentrations.
- However, some sub-populations (e.g., patients with renal impairment, patients with decreased muscle mass) may be at increased risk for reductions in serum carnitine concentrations during cefditoren pivoxil therapy. Furthermore, the appropriate dose in patients with end-stage renal disease has not been determined.
- As with other antibiotics, prolonged treatment may result in the possible emergence and overgrowth of resistant organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate alternative therapy should be administered.
- Cephalosporins may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated. In clinical trials, there was no difference between cefditoren and comparator cephalosporins in the incidence of increased prothrombin time.
Adverse Reactions
Clinical Trials Experience
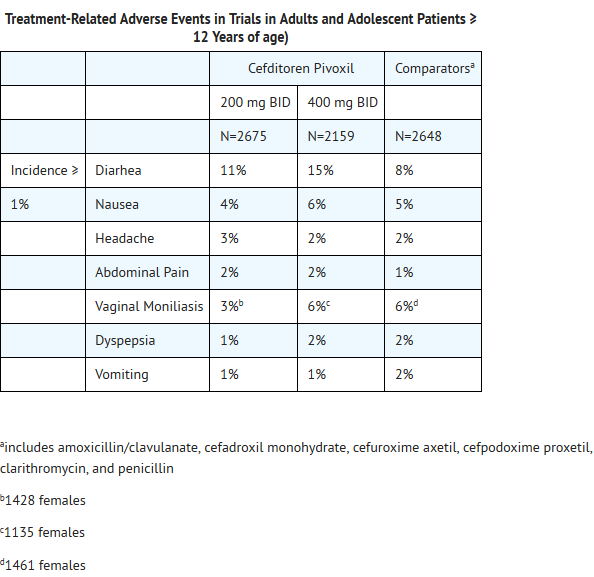
Clinical Trials – Cefditoren Pivoxil Tablets (Adults and Adolescent Patients ≥12 Years of Age)
- In clinical trials, 4834 adult and adolescent patients have been treated with the recommended doses of cefditoren pivoxil tablets (200 mg or 400 mg BID). Most adverse events were mild and self-limiting. No deaths or permanent disabilities have been attributed to cefditoren.
- The following adverse events were thought by the investigators to be possibly, probably, or definitely related to cefditoren tablets in multiple-dose clinical trials:
- The overall incidence of adverse events, and in particular diarrhea, increased with the higher recommended dose of Cefditoren Pivoxil.
- Treatment related adverse events experienced by <1% but >0.1% of patients who received 200 mg or 400 mg BID of cefditoren pivoxil were abnormal dreams, allergic reaction, anorexia, asthenia, asthma, coagulation time increased, constipation, dizziness, dry mouth, eructation, face edema, fever, flatulence, fungal infection, gastrointestinal disorder, hyperglycemia, increased appetite, insomnia, leukopenia, leukorrhea, liver function test abnormal, myalgia, nervousness, oral moniliasis, pain, peripheral edema, pharyngitis, pseudomembranous colitis, pruritus, rash, rhinitis, sinusitis, somnolence, stomatitis, sweating, taste perversion, thirst, thrombocythemia, urticaria, and vaginitis. Pseudomembranous colitis symptoms may begin during or after antibiotic treatment.
- Sixty-one of 2675 (2%) patients who received 200 mg BID and 69 of 2159 (3%) patients who received 400 mg BID of cefditoren pivoxil discontinued medication due to adverse events thought by the investigators to be possibly, probably, or definitely associated with cefditoren therapy. The discontinuations were primarily for gastrointestinal disturbances, usually diarrhea or nausea. Diarrhea was the reason for discontinuation in 19 of 2675 (0.7%) patients who received 200 mg BID and in 31 of 2159 (1.4%) patients who received 400 mg BID of cefditoren pivoxil.
- Changes in laboratory parameters of possible clinical significance, without regard to drug relationship and which occurred in ≥1% of patients who received cefditoren pivoxil 200 mg or 400 mg BID, were hematuria (3.0% and 3.1%), increased urine white blood cells (2.3% and 2.3%), decreased hematocrit (2.1% and 2.2%), and increased glucose (1.8% and 1.1%). Those events which occurred in <1% but >0.1% of patients included the following: increased/decreased white blood cells, increased eosinophils, decreased neutrophils, increased lymphocytes, increased platelet count, decreased hemoglobin, decreased sodium, increased potassium, decreased chloride, decreased inorganic phosphorus, decreased calcium, increased SGPT/ALT, increased SGOT/AST, increased cholesterol, decreased albumin, proteinuria, and increased BUN. It is not known if these abnormalities were caused by the drug or the underlying condition being treated.
- Cephalosporin Class Adverse Reactions
- In addition to the adverse reactions listed above which have been observed in patients treated with cefditoren pivoxil, the following adverse reactions and altered laboratory test results have been reported for cephalosporin class antibiotics:
- Adverse Reactions: Allergic reactions, anaphylaxis, drug fever, Stevens-Johnson syndrome, serum sickness-like reaction, erythema multiforme, toxic epidermal necrolysis, colitis, renal dysfunction, toxic nephropathy, reversible hyperactivity, hypertonia, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, hemorrhage, and superinfection.
- Altered Laboratory Tests: Prolonged prothrombin time, positive direct Coombs’ test, false-positive test for urinary glucose, elevated alkaline phosphatase, elevated bilirubin, elevated LDH, increased creatinine, pancytopenia, neutropenia, and agranulocytosis.
- Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Postmarketing Experience
- The following adverse experiences, regardless of their relationship to cefditoren pivoxil, have been reported during extensive postmarketing experience, beginning with approval in Japan in 1994: pneumonia interstitial, eosinophilic pneumonia acute, acute renal failure, arthralgia, thrombocytopenia, erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis and anaphylactoid reactions which may be accompanied by hypotension.
Drug Interactions
- Oral Contraceptives
- Multiple doses of cefditoren pivoxil had no effect on the pharmacokinetics of ethinyl estradiol, the estrogenic component in most oral contraceptives.
- Antacids
- Co-administration of a single dose of an antacid which contained both magnesium (800 mg) and aluminum (900 mg) hydroxides reduced the oral absorption of a single 400 mg dose of cefditoren pivoxil administered following a meal, as evidenced by a 14% decrease in mean Cmax and an 11% decrease in mean AUC. Although the clinical significance is not known, it is not recommended that cefditoren pivoxil be taken concomitantly with antacids.
- H2-Receptor Antagonists
- Co-administration of a single dose of intravenously administered famotidine (20 mg) reduced the oral absorption of a single 400 mg dose of cefditoren pivoxil administered following a meal, as evidenced by a 27% decrease in mean Cmax and a 22% decrease in mean AUC. Although the clinical significance is not known, it is not recommended that cefditoren pivoxil be taken concomitantly with H2 receptor antagonists.
- Probenecid
- As with other ß-lactam antibiotics, co-administration of probenecid with cefditoren pivoxil resulted in an increase in the plasma exposure of cefditoren, with a 49% increase in mean Cmax, a 122% increase in mean AUC, and a 53% increase in t1/2.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Cefditoren pivoxil was not teratogenic up to the highest doses tested in rats and rabbits. In rats, this dose was 1000 mg/kg/day, which is approximately 24 times a human dose of 200 mg BID based on mg/m2/day. In rabbits, the highest dose tested was 90 mg/kg/day, which is approximately four times a human dose of 200 mg BID based on mg/m2/day. This dose produced severe maternal toxicity and resulted in fetal toxicity and abortions.
- In a postnatal development study in rats, cefditoren pivoxil produced no adverse effects on postnatal survival, physical and behavioral development, learning abilities, and reproductive capability at sexual maturity when tested at doses of up to 750 mg/kg/day, the highest dose tested. This is approximately 18 times a human dose of 200 mg BID based on mg/m2/day.
- There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cefditoren in women who are pregnant.
Labor and Delivery
- Cefditoren pivoxil has not been studied for use during labor and delivery.
Nursing Mothers
- Cefditoren was detected in the breast milk of lactating rats. Because many drugs are excreted in human breast milk, caution should be exercised when cefditoren pivoxil is administered to nursing women.
Pediatric Use
- Use of cefditoren pivoxil is not recommended for pediatric patients less than 12 years of age. The safety and efficacy of cefditoren pivoxil tablets in this population, including any effects of altered carnitine concentration, have not been established.
Geriatic Use
- Of the 2675 patients in clinical studies who received cefditoren pivoxil 200 mg BID, 308 (12%) were >65 years of age. Of the 2159 patients in clinical studies who received cefditoren pivoxil 400 mg BID, 307 (14%) were >65 years of age. No clinically significant differences in effectiveness or safety were observed between older and younger patients. No dose adjustments are necessary in geriatric patients with normal (for their age) renal function. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Cefditoren with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cefditoren with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cefditoren in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cefditoren in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cefditoren in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cefditoren in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Cefditoren in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Cefditoren in the drug label.
Overdosage
Chronic Overdose
There is limited information regarding Chronic Overdose of Cefditoren in the drug label.
Pharmacology
| |
Cefditoren
| |
| Systematic (IUPAC) name | |
| (7R)-7-((Z)-2-(2-Aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-((Z)-2-(4-methylthiazol-5-yl)vinyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | J01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 506.58 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Cefditoren is a cephalosporin with antibacterial activity against gram-positive and gram-negative pathogens. The bactericidal activity of cefditoren results from the inhibition of cell wall synthesis via affinity for penicillin-binding proteins (PBPs).
Structure
- Cefditoren Pivoxil tablets contain cefditoren pivoxil, a semi-synthetic cephalosporin antibiotic for oral administration. It is a prodrug which is hydrolyzed by esterases during absorption, and the drug is distributed in the circulating blood as active cefditoren.
- Chemically, cefditoren pivoxil is (-)-(6R,7R)-2,2-dimethylpropionyloxymethyl 7-[(Z)-2-(2-aminothiazol-4-yl)-2-methoxy-iminoacetamido]-3-[(Z)-2-(4-methylthiazol-5-yl)ethenyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate. The empirical formula is C25H28N6O7S3 and the molecular weight is 620.73. The structural formula of cefditoren pivoxil is shown below:
- The amorphous form of cefditoren pivoxil developed for clinical use is a light yellow powder. It is freely soluble in dilute hydrochloric acid and soluble at levels equal to 6.06 mg/mL in ethanol and <0.1 mg/mL in water.
- Cefditoren Pivoxil tablets contain 200 mg or 400 mg of cefditoren as cefditoren pivoxil and the following inactive ingredients: croscarmellose sodium, mannitol, magnesium stearate, sodium caseinate (a milk protein), and sodium tripolyphosphate. The tablet coating contains carnauba wax, hypromellose, polyethylene glycol, and titanium dioxide. Tablets are printed with ink containing opacode blue S-1-10533.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Cefditoren in the drug label.
Pharmacokinetics
- Absorption
- Oral Bioavailability
- Following oral administration, cefditoren pivoxil is absorbed from the gastrointestinal tract and hydrolyzed to cefditoren by esterases. Maximal plasma concentrations (Cmax) of cefditoren under fasting conditions average 1.8 ± 0.6 µg/mL following a single 200 mg dose and occur 1.5 to 3 hours following dosing.
- Less than dose-proportional increases in Cmax and area under the concentration-time curve (AUC) were observed at doses of 400 mg and above. Cefditoren does not accumulate in plasma following twice daily administration to subjects with normal renal function. Under fasting conditions, the estimated absolute bioavailability of cefditoren pivoxil is approximately 14%. The absolute bioavailability of cefditoren pivoxil administered with a low fat meal (693 cal, 14 g fat, 122 g carb, 23 g protein) is 16.1 ± 3.0%.
- Food Effect
- Administration of cefditoren pivoxil following a high fat meal (858 cal, 64 g fat, 43 g carb, 31 g protein) resulted in a 70% increase in mean AUC and a 50% increase in mean Cmax compared to administration of cefditoren pivoxil in the fasted state. After a high fat meal, the Cmax averaged 3.1 ± 1.0 µg/mL following a single 200 mg dose of cefditoren pivoxil and 4.4 ± 0.9 µg/mL following a 400 mg dose. Cefditoren AUC and Cmax values from studies conducted with a moderate fat meal (648 cal, 27 g fat, 73 g carb, 29 g protein) are similar to those obtained following a high fat meal.
- Distribution
- The mean volume of distribution at steady state (Vss) of cefditoren is 9.3 ± 1.6 L. Binding of cefditoren to plasma proteins averages 88% from in vitro determinations, and is concentration-independent at cefditoren concentrations ranging from 0.05 to10 µg/mL. Cefditoren is primarily bound to human serum albumin and its binding is decreased when serum albumin concentrations are reduced. Binding to α-1-acid glycoprotein ranges from 3.3 to 8.1%. Penetration into red blood cells is negligible.
- Skin blister fluid
- Maximal concentrations of cefditoren in suction-induced blister fluid were observed 4 to 6 hours following administration of a 400 mg dose of cefditoren pivoxil with a mean of 1.1 ± 0.42 µg/mL. Mean blister fluid AUC values were 56 ± 15% of corresponding plasma concentrations.
- Tonsil tissue
- In fasted patients undergoing elective tonsillectomy, the mean concentration of cefditoren in tonsil tissue 2 to 4 hours following administration of a 200 mg dose of cefditoren pivoxil was 0.18 ± 0.07 µg/g. Mean tonsil tissue concentrations of cefditoren were 12 ± 3% of the corresponding serum concentrations.
- Cerebrospinal Fluid (CSF)
- Data on the penetration of cefditoren into human cerebrospinal fluid are not available.
- Metabolism and Excretion
- Cefditoren is eliminated from the plasma, with a mean terminal elimination half-life (t1/2) of 1.6 ± 0.4 hours in young healthy adults. Cefditoren is not appreciably metabolized. After absorption, cefditoren is mainly eliminated by excretion into the urine, with a renal clearance of approximately 4-5 L/h. Studies with the renal tubular transport blocking agent probenecid indicate that tubular secretion, along with glomerular filtration is involved in the renal elimination of cefditoren. Cefditoren renal clearance is reduced in patients with renal insufficiency.
- Hydrolysis of cefditoren pivoxil to its active component, cefditoren, results in the formation of pivalate. Following multiple doses of cefditoren pivoxil, greater than 70% of the pivalate is absorbed. Pivalate is mainly eliminated (>99%) through renal excretion, nearly exclusively as pivaloylcarnitine. Following a 200 mg BID regimen for 10 days, the mean decrease in plasma concentrations of total carnitine was 18.1 ± 7.2 nmole/mL, representing a 39% decrease in plasma carnitine concentrations. Following a 400 mg BID regimen for 14 days, the mean decrease in plasma concentrations of carnitine was 33.3 ± 9.7 nmole/mL, representing a 63% decrease in plasma carnitine concentrations. Plasma concentrations of carnitine returned to the normal control range within 7 to 10 days after discontinuation of cefditoren pivoxil.
- Special Populations
- Geriatric
- The effect of age on the pharmacokinetics of cefditoren was evaluated in 48 male and female subjects aged 25 to 75 years given 400 mg cefditoren pivoxil BID for 7 days. Physiological changes related to increasing age increased the extent of cefditoren exposure in plasma, as evidenced by a 26% higher Cmax and a 33% higher AUC for subjects aged ≥ 65 years compared with younger subjects. The rate of elimination of cefditoren from plasma was lower in subjects aged ≥ 65 years, with t1/2 values 16-26% longer than for younger subjects. Renal clearance of cefditoren in subjects aged ≥ 65 years was 20-24% lower than in younger subjects. These changes could be attributed to age-related changes in creatinine clearance. No dose adjustments are necessary for elderly patients with normal (for their age) renal function.
- Gender
- The effect of gender on the pharmacokinetics of cefditoren was evaluated in 24 male and 24 female subjects given 400 mg cefditoren pivoxil BID for 7 days. The extent of exposure in plasma was greater in females than in males, as evidenced by a 14% higher Cmax and a 16% higher AUC for females compared to males. Renal clearance of cefditoren in females was 13% lower than in males. These differences could be attributed to gender-related differences in lean body mass. No dose adjustments are necessary for gender.
- Renal Insufficiency
- Cefditoren pharmacokinetics were investigated in 24 adult subjects with varying degrees of renal function following administration of cefditoren pivoxil 400 mg BID for 7 days. Decreased creatinine clearance (CLcr) was associated with an increase in the fraction of unbound cefditoren in plasma and a decrease in the cefditoren elimination rate, resulting in greater systemic exposure in subjects with renal impairment. The unbound Cmax and AUC were similar in subjects with mild renal impairment (CLcr: 50-80 mL/min/1.73 m2) compared to subjects with normal renal function (CLcr: >80 mL/min/1.73 m2). Moderate (CLcr: 30-49 mL/min/1.73 m2) or severe (CLcr: <30 mL/min/1.73 m2) renal impairment increased the extent of exposure in plasma, as evidenced by mean unbound Cmax values 90% and 114% higher and AUC values 232% and 324% higher than that for subjects with normal renal function. The rate of elimination from plasma was lower in subjects with moderate or severe renal impairment, with respective mean t1/2 values of 2.7 and 4.7 hours. No dose adjustment is necessary for patients with mild renal impairment (CLcr: 50-80 mL/min/1.73 m2). It is recommended that not more than 200 mg BID be administered to patients with moderate renal impairment (CLcr: 30-49 mL/min/1.73 m2) and 200 mg QD be administered to patients with severe renal impairment (CLcr: <30 mL/min/1.73 m2).
- Hemodialysis
- Cefditoren pharmacokinetics investigated in six adult subjects with end-stage renal disease (ESRD) undergoing hemodialysis given a single 400 mg dose of cefditoren pivoxil were highly variable. The mean t1/2 was 4.7 hours and ranged from 1.5 to 15 hours. Hemodialysis (4 hours duration) removed approximately 30% of cefditoren from systemic circulation but did not change the apparent terminal elimination half-life. The appropriate dose for ESRD patients has not been determined.
- Hepatic Disease
- Cefditoren pharmacokinetics were evaluated in six adult subjects with mild hepatic impairment (Child-Pugh Class A) and six with moderate hepatic impairment (Child-Pugh Class B). Following administration of cefditoren pivoxil 400 mg BID for 7 days in these subjects, mean Cmax and AUC values were slightly (<15%) greater than those observed in normal subjects. No dose adjustments are necessary for patients with mild or moderate hepatic impairment (Child-Pugh Class A or B). The pharmacokinetics of cefditoren in subjects with severe hepatic impairment (Child-Pugh Class C) have not been studied.
Nonclinical Toxicology
- No long-term animal carcinogenicity studies have been conducted with cefditoren pivoxil. Cefditoren pivoxil was not mutagenic in the Ames bacterial reverse mutation assay, or in the mouse lymphoma mutation assay at the hypoxanthineguanine phosphoribosyltransferase locus. In Chinese hamster lung cells, chromosomal aberrations were produced by cefditoren pivoxil, but not by cefditoren. Subsequent studies showed that the chromosome aberrations were due to the release of formaldehyde from the pivoxil ester moiety in the in vitro assay system. Neither cefditoren nor cefditoren pivoxil produced chromosomal aberrations when tested in an in vitro human peripheral blood lymphocyte assay, or in the in vivo mouse micronucleus assay. Cefditoren pivoxil did not induce unscheduled DNA syntheses when tested. In rats, fertility and reproduction were not affected by cefditoren pivoxil at oral doses up to 1000 mg/kg/day, approximately 24 times a human dose of 200 mg BID based on mg/m2/day.
Clinical Studies
There is limited information regarding Clinical Studies of Cefditoren in the drug label.
How Supplied
- Cefditoren Pivoxil tablets containing cefditoren pivoxil equivalent to 200 mg or 400 mg of cefditoren are available as white, elliptical, film-coated tablets imprinted with “CBP 200” or “CBP 400” in blue. These tablets are available in blister packages, as follows:
- NDC 44009-802-20: 400 mg 20 count blister pack. Cefditoren Pivoxil tablets containing cefditoren pivoxil equivalent to 400 mg of cefditoren are available as white, elliptical, film-coated tablets imprinted with “CBP 400” in blue.
- NDC 44009-802-28: 400 mg 28 count blister pack. Cefditoren Pivoxil tablets containing cefditoren pivoxil equivalent to 400 mg of cefditoren are available as white, elliptical, film-coated tablets imprinted with “CBP 400” in blue.
- NDC 44009-801-20: 20 count blister pack. Cefditoren Pivoxil tablets containing cefditoren pivoxil equivalent to 200 mg of cefditoren are available as white, elliptical, film-coated tablets imprinted with “CBP 200” in blue.
Storage
There is limited information regarding Cefditoren Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Cefditoren |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cefditoren |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be counseled that antibacterial drugs including Cefditoren Pivoxil should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefditoren Pivoxil is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefditoren Pivoxil or other antibacterial drugs in the future.
- Cefditoren Pivoxil should be taken with meals to enhance absorption.
- Cefditoren Pivoxil may be taken concomitantly with oral contraceptives.
- It is not recommended that Cefditoren Pivoxil be taken concomitantly with antacids or other drugs taken to reduce stomach acids.
- Cefditoren Pivoxil tablets contain sodium caseinate, a milk protein. Patients with milk protein hypersensitivity (not lactose intolerance) should not be administered Cefditoren Pivoxil.
Precautions with Alcohol
- Alcohol-Cefditoren interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CEFDITOREN PIVOXIL®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "CEFDITOREN PIVOXIL cefditoren pivoxil tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Cefditoren
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Cefditoren |Label Name=Cefditoren03.png
}}
{{#subobject:
|Label Page=Cefditoren |Label Name=Cefditoren04.png
}}