Cathepsin G
| Cathepsin G | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1au8. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | CTSG ; CG; MGC23078 | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 20450 | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
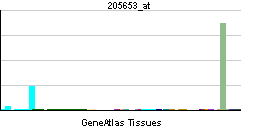 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Cathepsin G, also known as CTSG, is a human gene.
The protein encoded by this gene, a member of the peptidase S1 protein family, is found in azurophil granules of neutrophilic polymorphonuclear leukocytes. The encoded protease has a specificity similar to that of chymotrypsin C, and may participate in the killing and digestion of engulfed pathogens, and in connective tissue remodeling at sites of inflammation. Transcript variants utilizing alternative polyadenylation signals exist for this gene.[1]
References
Further reading
- Shafer WM, Katzif S, Bowers S; et al. (2002). "Tailoring an antibacterial peptide of human lysosomal cathepsin G to enhance its broad-spectrum action against antibiotic-resistant bacterial pathogens". Curr. Pharm. Des. 8 (9): 695–702. PMID 11945165.
- Cohen AB, Stevens MD, Miller EJ; et al. (1992). "Generation of the neutrophil-activating peptide-2 by cathepsin G and cathepsin G-treated human platelets". Am. J. Physiol. 263 (2 Pt 1): L249–56. PMID 1387511.
- Sasaki T, Ueno-Matsuda E (1993). "Immunocytochemical localization of cathepsins B and G in odontoclasts of human deciduous teeth". J. Dent. Res. 71 (12): 1881–4. PMID 1452887.
- Maison CM, Villiers CL, Colomb MG (1991). "Proteolysis of C3 on U937 cell plasma membranes. Purification of cathepsin G.". J. Immunol. 147 (3): 921–6. PMID 1861080.
- Brandt E, Van Damme J, Flad HD (1991). "Neutrophils can generate their activator neutrophil-activating peptide 2 by proteolytic cleavage of platelet-derived connective tissue-activating peptide III". Cytokine. 3 (4): 311–21. PMID 1873479.
- Kargi HA, Campbell EJ, Kuhn C (1990). "Elastase and cathepsin G of human monocytes: heterogeneity and subcellular localization to peroxidase-positive granules". J. Histochem. Cytochem. 38 (8): 1179–86. PMID 2164060.
- Pratt CW, Tobin RB, Church FC (1990). "Interaction of heparin cofactor II with neutrophil elastase and cathepsin G.". J. Biol. Chem. 265 (11): 6092–7. PMID 2318847.
- Gabay JE, Scott RW, Campanelli D; et al. (1989). "Antibiotic proteins of human polymorphonuclear leukocytes". Proc. Natl. Acad. Sci. U.S.A. 86 (14): 5610–4. PMID 2501794.
- Hohn PA, Popescu NC, Hanson RD; et al. (1989). "Genomic organization and chromosomal localization of the human cathepsin G gene". J. Biol. Chem. 264 (23): 13412–9. PMID 2569462.
- Livesey SA, Buescher ES, Krannig GL; et al. (1990). "Human neutrophil granule heterogeneity: immunolocalization studies using cryofixed, dried and embedded specimens". Scanning Microsc. Suppl. 3: 231–9, discussion 239-40. PMID 2616953.
- Campbell EJ, Silverman EK, Campbell MA (1989). "Elastase and cathepsin G of human monocytes. Quantification of cellular content, release in response to stimuli, and heterogeneity in elastase-mediated proteolytic activity". J. Immunol. 143 (9): 2961–8. PMID 2681419.
- Salvesen G, Farley D, Shuman J; et al. (1987). "Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases". Biochemistry. 26 (8): 2289–93. PMID 3304423.
- Heck LW, Rostand KS, Hunter FA, Bhown A (1987). "Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil cathepsin G from normal donors". Anal. Biochem. 158 (1): 217–27. PMID 3799965.
- Crocker J, Jenkins R, Burnett D (1986). "Immunohistochemical localization of cathepsin G in human tissues". Am. J. Surg. Pathol. 9 (5): 338–43. PMID 3911778.
- Klickstein LB, Kaempfer CE, Wintroub BU (1983). "The granulocyte-angiotensin system. Angiotensin I-converting activity of cathepsin G.". J. Biol. Chem. 257 (24): 15042–6. PMID 6294088.
- LaRosa CA, Rohrer MJ, Benoit SE; et al. (1994). "Neutrophil cathepsin G modulates the platelet surface expression of the glycoprotein (GP) Ib-IX complex by proteolysis of the von Willebrand factor binding site on GPIb alpha and by a cytoskeletal-mediated redistribution of the remainder of the complex". Blood. 84 (1): 158–68. PMID 7517206.
- Owen CA, Campbell MA, Sannes PL; et al. (1995). "Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases". J. Cell Biol. 131 (3): 775–89. PMID 7593196.
- Savage MJ, Iqbal M, Loh T; et al. (1994). "Cathepsin G: localization in human cerebral cortex and generation of amyloidogenic fragments from the beta-amyloid precursor protein". Neuroscience. 60 (3): 607–19. PMID 7936190.
- Grisolano JL, Sclar GM, Ley TJ (1994). "Early myeloid cell-specific expression of the human cathepsin G gene in transgenic mice". Proc. Natl. Acad. Sci. U.S.A. 91 (19): 8989–93. PMID 8090757.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. PMID 8125298.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |
This article incorporates text from the United States National Library of Medicine, which is in the public domain.