Carcinoid syndrome pathophysiology
|
Carcinoid syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Carcinoid syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Carcinoid syndrome pathophysiology |
|
Risk calculators and risk factors for Carcinoid syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]Associate Editor(s)-in-Chief: Parminder Dhingra, M.D. [3]
Overview
- Carcinoid syndrome (CS) is a paraneoplastic syndrome caused by the secretion of serotonin (5-hydroxytrptamine)(primary marker) but can be caused by secretion of histamine, kallikrein, prostaglandins, and tachykinins..*Carcinoid syndrome is most commonly caused by neuroendocrine tumors of midgut.70% of tryptophan is converted into serotonin which leads to secondary deficiency of niacin.*Serotonin metabolizes into 5-hydroxy indoleacetic acid (5-HIAA) by aldehyde dehydrogenase, which is eliminated into the urine.
Pathophysiology
- Carcinoid syndrome (CS) is a paraneoplastic syndrome associated with the secretion of several humoral factors.[1]
- The primary marker is serotonin (5-hydroxytrptamine) but can be caused by secretion of histamine, kallikrein, prostaglandins, and tachykinins.
- Carcinoid syndrome is most commonly caused by neuroendocrine tumors of midgut.
- Serotonin and kallikrein are inactivated in the liver and manifestations of carcinoid syndrome do not occur until there are metastases to the liver.
- Exceptions include circumstances in which venous blood draining from a carcinoid tumor enters directly into the systemic circulation which are followings:
- Primary pulmonary or ovarian carcinoid
- Pelvic or retroperitoneal involvement by metastatic or locally invasive small bowel carcinoid.
- Extensive bone metastases
- Only 1% of dietary tryptophan is converted into serotonin. However, in a patient with neuroendocrine tumors, up to 70% of tryptophan is converted into serotonin.
- Serotonin undergoes oxidative reaction and leads to the formation of 5-hydroxy indoleacetic acid (5-HIAA) by aldehyde dehydrogenase, which is eliminated into the urine.
- Serotonin causes increased motility and secretions resulting in diarrhea.
- As most of the body's tryptophan is diverted to serotonin formation pathway by neuroendocrine tumors, it leads to a deficiency of tryptophan which is needed for synthesis of niacin.
- Deficiency of niacin results in Pellagra which manifests as dermatitis, dementia, and diarrhea.
- Prostaglandins also mediate increased intestinal motility and fluid secretion in GI tract causing diarrhea.
- The flushing results from secretion of kallikrein, the enzyme that catalyzes the conversion of kininogen to lysyl-bradykinin.
- Lysyl-bradykinin is further converted to bradykinin, a strong vasodilator.
- Large amounts of serotonin produces pellagra-like features including diarrhea.
Lung Carcinoid Tumor
- Carcinoid tumors arising in the bronchi reach the systemic circulation before passing through the liver and may be associated with bronchoconstriction and manifestations of carcinoid syndrome without liver metastases.
- Bronchospasm leading to wheezing is caused by release of histamin and serotonin.[2]
- Episodes of flushing and related manifestations are particularly prolonged or severe if carcinoid syndrome is caused by a lung neuroendocrine tumour.
Carcinoid Heart Disease
- 5-HT2B is the receptor of serotonin in the cardiovascular system that may be involved in fibrogenesis.[3]
- Activation of the 5-HT2B receptor triggers distinct intracellular signaling pathways, which in turn may result in a stronger inflammatory response and release of cytokines including TNF-alpha, activation of the MAPK signaling pathway and hyperexpression of TGF-beta leading to to cardiac fibrosis.[4][5][6]
- Fibrosis leads to thickening of mural and valvular endothelial surfaces of right-sided cardiac structures.[7][8]
- Fibrosis leads to
- Tricuspid and pulmonic regurgitation.
- Pulmonary stenosis.
- Mitral and aortic insufficiency.
- Cardiac dysrhythmias
Mesentric fibrosis
- Serotonin and TGF-beta secreted by neuroendocrine tumours appears to play a central role in the development of mesentric fibrosis.[9]
- It is another complication of uncontrolled carcinoid syndrome.
- There is a fibrotic and desmoplastic reaction around metastatic mesenteric lymph nodes.
- Mesentric fibrosis is a pathognomonic radiological sign of midgut NET, which can be observed on computerized tomography and nuclear magnetic resonance images.
- Mesentric fibrosis can lead to ischemia of vessels and intestinal obstruction.
- This vascular ischemia can lead to bowel congestion and result in decreased absorption of nutrients and can also cause ascites and more severe cases of mesenteric ischemia.[10][11]
Genetics
- Gastrointestinal carcinoids occur in association with inherited syndromes, such as multiple endocrine neoplasia type 1 and neurofibromatosis type 1.[12]
- Multiple endocrine neoplasia type 1 is caused by alterations of the MEN1 gene located at chromosomal region 11q13.
- Most carcinoids associated with multiple endocrine neoplasia type 1 appear to be of foregut origin.
- Neurofibromatosis type 1 is an autosomal dominant genetic disorder caused by alteration of the NF1 gene at chromosome 17q11.
- Carcinoids in patients with neurofibromatosis type 1 appear to arise primarily in the periampullary region.[13]
- A hereditary form of small intestinal cacinoid tumour has been found which is caused by mutation in the IPMK gene leads to higher risk of developing carcinoid tumors in the small intestine.[14]
- The most frequently reported mutated gene in gastrointestinal carcinoids is β-catenin (CTNNB1).[15]
Embryology
- Carcinoid tumors originate from neuroendocrine cells (enterochromaffin or amine precursor uptake and decarboxylase [APUD] cells) which are derived from neural crest cells embrologically.
- Gastrointestinal carcinoids derive from cells that migrate from the neural crest to the foregut, midgut and hindgut.[16]
Location
Carcinoid tumors are normally found throughout the gastrointestinal tract from mouth to anus, with the highest concentration of cells in the appendix and small intestine. The pancreas contains a large number of these cells, the biliary tree only a few and the liver normally contains none. Fibrotic lesions are found on endocardium, particularly on the right side of the heart.
Gross Pathology
Gastrointestinal Carcinoid
In the gastric or intestinal wall, carcinoids may occur as firm white, yellow, or gray nodules and may be intramural masses or may protrude into the lumen as polypoid nodules. The overlying gastric or intestinal mucosa may be intact or have focal ulceration.
- Well-differentiated NETs of the tubular gastrointestinal tract are often well-circumscribed round lesions in the submucosa or extending to the muscular layer.
- The cut surface appears red to tan, reflecting the abundant microvasculature, or sometimes yellow because of high lipid content.
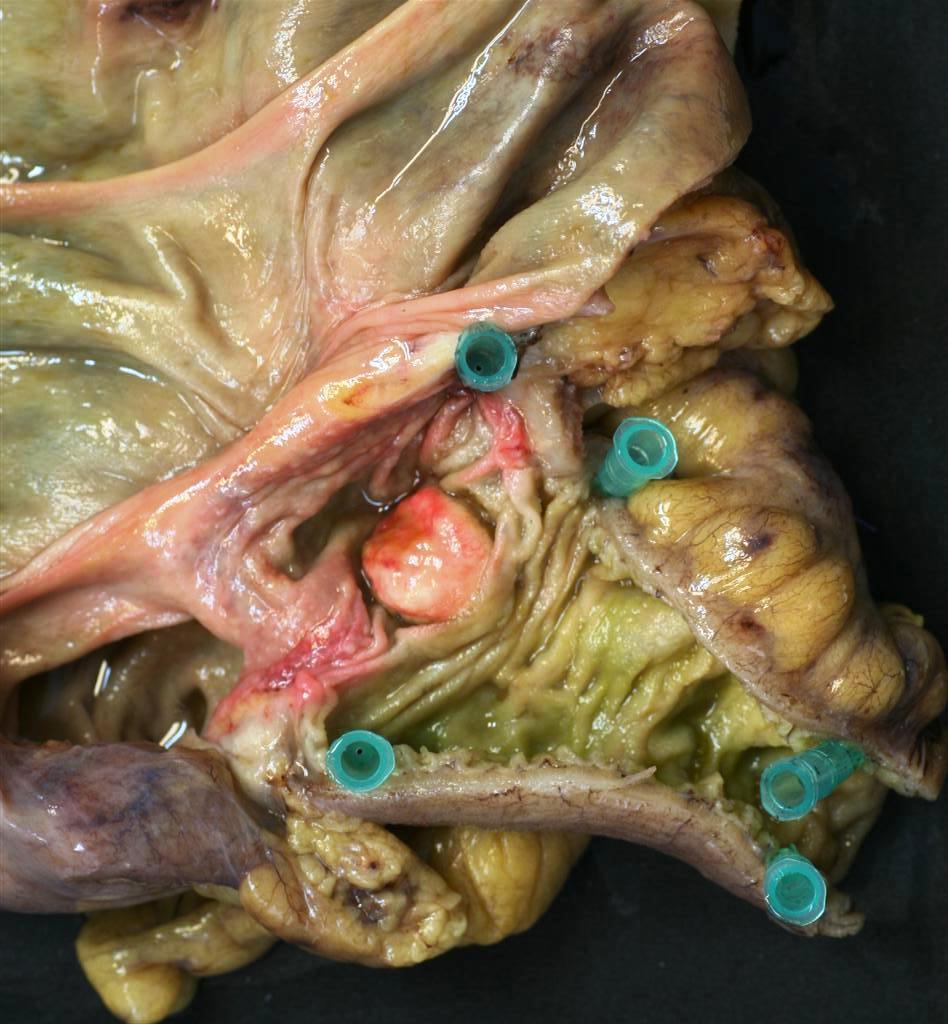 |
Neuroendocrine tumours of the lung
- Pulmonary neoplasms that are characterized by neuroendocrine differentiation and relatively indolent clinical behavior.
- Lung is the second most common site for neuroendocrine tumours.
- Lung NET are classified on the basis of histology:
- Typical neuroendocrine tumours :well-differentiated, low-grade, slowly growing neoplasms that seldom metastasize to extrathoracic structures and localized.
- Poorly differentiated and high-grade neuroendocrine carcinomas, as typified by small cell lung cancer and large cell carcinomas which behaves aggressively, with rapid tumor growth and early distant dissemination.
- Atypical neuroendorcrine tumors, which are of intermediate grade and differentiation, is intermediate between typical neuroendocrine tumors and small cell lung cancer.
- Based on the location:
Carcinoid tumor of the lung may be classified based on the location into two subtypes:
- Bronchial carcinoid tumors: central lesions
- Peripheral pulmonary carcinoid tumors: peripheral lesions
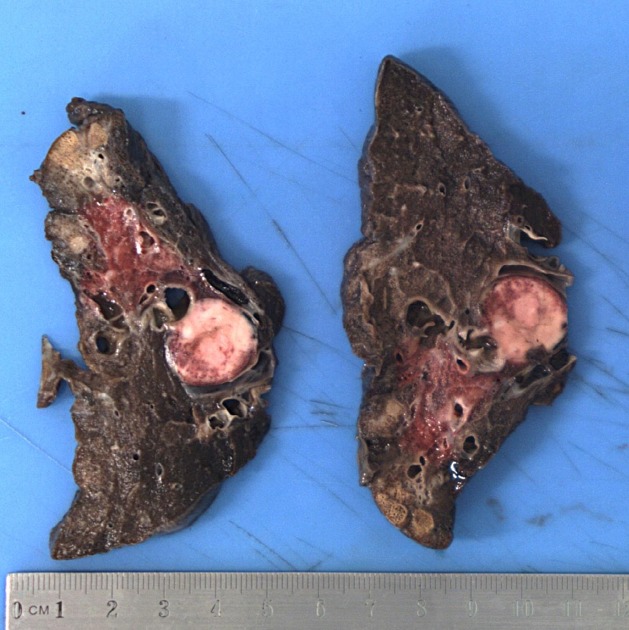
Microscopic Pathology
- NETs arise from enterochromaffin (neuroendocrine) cells of the aerodigstive tract.
- The term enterochromaffin refers to the ability to stain with potassium chromate (chromaffin), a feature of cells that contain serotonin.
- On electron microscopy ,the cells in tumors are found to contain membrane-bound secretory granules with dense-core granules in the cytoplasm.
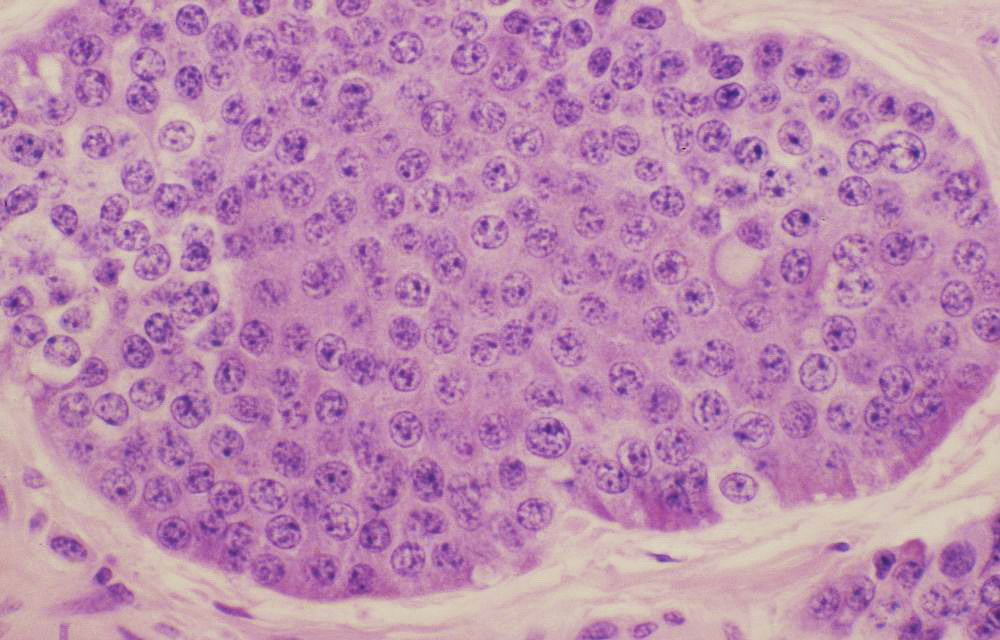
Typical carcinoid histopathology-The nuclei of the tumor cells are uniform with a stippled chromatin pattern. There is no mitotic activity or necrosis. https://commons.wikimedia.org/wiki/File:Typical_carcinoid_(3931156341).jpg source Case courtesy of Dr Yale Rosen, from wikicommons - The most recent nomenclature for NETs of the digestive system from the World Health Organization (WHO) distinguishes two broad subgroups:[18][19][20]
- Well-differentiated NETs: which are further subdivided according to proliferative rate:
- Low grade
- Intermediate grade.(Intermediate-grade NETs arising in the lung (but not elsewhere) are referred to as atypical carcinoids
- Poorly differentiated neuroendocrine carcinomas
- They are high-grade carcinomas that resemble small cell or large cell neuroendocrine carcinoma of the lung.
- Histoloigically, well-differentiated NETs have characteristic "organoid" arrangements of tumor cells, with solid/nesting, trabecular, gyriform, or sometimes, glandular patterns.
- The cells are relatively uniform, and they have round to oval nuclei, coarsely stippled chromatin, and finely granular cytoplasm.
- The cells produce abundant neurosecretory granules, as reflected in the strong and diffuse immunohistochemical expression of neuroendocrine markers such as synaptophysin and chromogranin.
- Well-differentiated NETs of the midgut (ileum in particular) also have a very characteristic pattern of solid or cribriform nests punctuated by sharply outlined luminal spaces with peripheral nuclear palisading and granular eosinophilic cytoplasm.
- Poorly differentiated neuroendocrine carcinomas (NECs) less closely resemble nonneoplastic neuroendocrine cells and have a more sheet-like or diffuse architecture, irregular nuclei, and less cytoplasmic granularity. Immunohistochemical expression of neuroendocrine markers is generally more limited in extent and intensity.
References
- ↑ Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP (August 2018). "Carcinoid syndrome: update on the pathophysiology and treatment". Clinics (Sao Paulo). 73 (suppl 1): e490s. doi:10.6061/clinics/2018/e490s. PMC 6096975. PMID 30133565.
- ↑ Kvols LK, Moertel CG, O'Connell MJ, Schutt AJ, Rubin J, Hahn RG (September 1986). "Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue". N. Engl. J. Med. 315 (11): 663–6. doi:10.1056/NEJM198609113151102. PMID 2427948.
- ↑ Grozinsky-Glasberg S, Grossman AB, Gross DJ (2015). "Carcinoid Heart Disease: From Pathophysiology to Treatment--'Something in the Way It Moves'". Neuroendocrinology. 101 (4): 263–73. doi:10.1159/000381930. PMID 25871411.
- ↑ Launay JM, Birraux G, Bondoux D, Callebert J, Choi DS, Loric S, Maroteaux L (February 1996). "Ras involvement in signal transduction by the serotonin 5-HT2B receptor". J. Biol. Chem. 271 (6): 3141–7. PMID 8621713.
- ↑ Jaffré F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L (January 2009). "Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy". Circ. Res. 104 (1): 113–23. doi:10.1161/CIRCRESAHA.108.180976. PMID 19023134.
- ↑ Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B (December 2002). "Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells". Am. J. Pathol. 161 (6): 2209–18. doi:10.1016/S0002-9440(10)64497-5. PMID 12466135.
- ↑ Carcinoid cardiac lesions. Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia. http://radiopaedia.org/articles/carcinoid-cardiac-lesions
- ↑ Luis SA, Pellikka PA (January 2016). "Carcinoid heart disease: Diagnosis and management". Best Pract. Res. Clin. Endocrinol. Metab. 30 (1): 149–58. doi:10.1016/j.beem.2015.09.005. PMID 26971851.
- ↑ Druce MR, Bharwani N, Akker SA, Drake WM, Rockall A, Grossman AB (March 2010). "Intra-abdominal fibrosis in a recent cohort of patients with neuroendocrine ('carcinoid') tumours of the small bowel". QJM. 103 (3): 177–85. doi:10.1093/qjmed/hcp191. PMID 20123681.
- ↑ Pantongrag-Brown L, Buetow PC, Carr NJ, Lichtenstein JE, Buck JL (February 1995). "Calcification and fibrosis in mesenteric carcinoid tumor: CT findings and pathologic correlation". AJR Am J Roentgenol. 164 (2): 387–91. doi:10.2214/ajr.164.2.7839976. PMID 7839976.
- ↑ Daskalakis K, Karakatsanis A, Stålberg P, Norlén O, Hellman P (January 2017). "Clinical signs of fibrosis in small intestinal neuroendocrine tumours". Br J Surg. 104 (1): 69–75. doi:10.1002/bjs.10333. PMID 27861745.
- ↑ General Information About Gastrointestinal (GI) Carcinoid Tumors.<ref name="pmid2886072">Duh QY, Hybarger CP, Geist R, Gamsu G, Goodman PC, Gooding GA, Clark OH (July 1987). "Carcinoids associated with multiple endocrine neoplasia syndromes". Am. J. Surg. 154 (1): 142–8. PMID 2886072.
- ↑ Karatzas G, Kouraklis G, Karayiannakis A, Patapis P, Givalos N, Kaperonis E (June 2000). "Ampullary carcinoid and jejunal stromal tumour associated with von Recklinghausen's disease presenting as gastrointestinal bleeding and jaundice". Eur J Surg Oncol. 26 (4): 428–9. doi:10.1053/ejso.1999.0911. PMID 10873367.
- ↑ Sei Y, Zhao X, Forbes J, Szymczak S, Li Q, Trivedi A, Voellinger M, Joy G, Feng J, Whatley M, Jones MS, Harper UL, Marx SJ, Venkatesan AM, Chandrasekharappa SC, Raffeld M, Quezado MM, Louie A, Chen CC, Lim RM, Agarwala R, Schäffer AA, Hughes MS, Bailey-Wilson JE, Wank SA (July 2015). "A Hereditary Form of Small Intestinal Carcinoid Associated With a Germline Mutation in Inositol Polyphosphate Multikinase". Gastroenterology. 149 (1): 67–78. doi:10.1053/j.gastro.2015.04.008. PMC 4858647. PMID 25865046.
- ↑ Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T (September 2001). "Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin gene in gastrointestinal carcinoid tumor". Cancer Res. 61 (18): 6656–9. PMID 11559529.
- ↑ Reznek RH (2006). "CT/MRI of neuroendocrine tumours". Cancer Imaging. 6: S163–77. doi:10.1102/1470-7330.2006.9037. PMC 1805060. PMID 17114072.
- ↑ Image courtesy of Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia (original file [1]). [http://radiopaedia.org/licence Creative Commons BY-SA-NC
- ↑ Schott M, Klöppel G, Raffel A, Saleh A, Knoefel WT, Scherbaum WA (May 2011). "Neuroendocrine neoplasms of the gastrointestinal tract". Dtsch Arztebl Int. 108 (18): 305–12. doi:10.3238/arztebl.2011.0305. PMC 3103981. PMID 21629514.
- ↑ Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML (August 2017). "Role of PD-L1 expression as a biomarker for GEP neuroendocrine neoplasm grading". Cell Death Dis. 8 (8): e3004. doi:10.1038/cddis.2017.401. PMC 5596583. PMID 28837143.
- ↑ Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, Balci S, Gucer H, Jang KT, Tajiri T, Basturk O, Kong SY, Goodman M, Akkas G, Adsay V (May 2015). "Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies". Mod. Pathol. 28 (5): 686–94. doi:10.1038/modpathol.2014.156. PMC 4460192. PMID 25412850.