Carcinoid syndrome pathophysiology
|
Carcinoid syndrome Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Carcinoid syndrome pathophysiology On the Web |
|
American Roentgen Ray Society Images of Carcinoid syndrome pathophysiology |
|
Risk calculators and risk factors for Carcinoid syndrome pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]Associate Editor(s)-in-Chief: Parminder Dhingra, M.D. [3]
Overview
The pathophysiology of carcinoid tumor depends on the histological subtype. genes in the pathogenesis of carcinoid tumor are β-catenin, NF1, and MEN1. Carcinoid tumors originate from neuroendocrine cells. On microscopic histopathological analysis, gastrointestinal carcinoid syndrome is characterized by solid or small trabecular clusters of neuroendocrine cells with uniform nuclei and abundant granular or faintly staining (clear) cytoplasm.
Pathophysiology
- Carcinoid syndrome (CS) is a paraneoplastic syndrome associated with the secretion of several humoral factors such as polypeptides, vasoactive amines, and prostaglandins from the well-differentiated neuroendocrine tumors
- The primary marker is serotonin (5-HT) but can be caused by secretion of histamine, kallikrein, prostaglandins, and tachykinins.
- Carcinoid syndrome is most commonly caused by neuroendocrine tumors of midgut.
- Serotonin and kallikrein are inactivated in the liver and manifestations of carcinoid syndrome do not occur until there are metastases to the liver.
- Exceptions include circumstances in which venous blood draining from a carcinoid tumor enters directly into the systemic circulation which are followings:
- Primary pulmonary or ovarian carcinoids
- Pelvic or retroperitoneal involvement by metastatic or locally invasive small bowel carcinoids
- Extensive bone metastases
- Only 1% of dietary tryptophan is converted into serotonin. However, in a patient with neuroendocrine tumors, up to 70% of tryptophan is converted into serotonin.
- Serotonin undergoes oxidative reaction and leads to the formation of 5-hydroxy indoleacetic acid (5-HIAA) by aldehyde dehydrogenase, which is eliminated into the urine.
- Serotonin causes increased motility and secretions resulting in diarrhea.
- As most of the body's tryptophan is diverted to serotonin formation pathway by neuroendocrine tumors, it leads to a deficiency of tryptophan which is needed for synthesis of niacin.
- Deficiency of niacin results in Pellagra which manifests as dermatitis, dementia, and diarrhea.
- Prostaglandins also mediate increased intestinal motility and fluid secretion in GI tract causing diarrhea
- The flushing results from secretion of kallikrein, the enzyme that catalyzes the conversion of kininogen to lysyl-bradykinin.
- Lysyl-bradykinin is further converted to bradykinin, a strong vasodilator.
- Large amounts of serotonin produces pellagra-like features including diarrhea.
Lung Carcinoid Tumor
- Carcinoid tumors arising in the bronchi reach the systemic circulation before passing through the liver and may be associated with bronchoconstriction and manifestations of carcinoid syndrome without liver metastases.
- Bronchospasm leading to wheezing is caused by release of histamin and serotonin.[1]
Carcinoid Heart Disease
- 5-HT2B is the receptor of serotonin in the cardiovascular system that may be involved in fibrogenesis.
- Activation of the 5-HT2B receptor triggers distinct intracellular signaling pathways, which in turn may result in a stronger inflammatory response and release of cytokines including TNF-alpha, activation of the MAPK signaling pathway and hyperexpression of TGF-beta leading to to cardiac fibrosis.[2][3][4]
- Fibrosis leads to thickening of mural and valvular endothelial surfaces of right-sided cardiac structures.[5][6]
- Fibrosis leads to
- Tricuspid and pulmonic regurgitation.
- Pulmonary stenosis.
- Mitral and aortic insufficiency.
- Cardiac dysrhythmias
Mesentric fibrosis
- Serotonin and TGF-beta secreted by neuroendocrine tumours appears to play a central role in the development of mesentric fibrosis.[7]
Genetics
- Gastrointestinal carcinoids occur in association with inherited syndromes, such as multiple endocrine neoplasia type 1 and neurofibromatosis type 1.[8]
- Multiple endocrine neoplasia type 1 is caused by alterations of the MEN1 gene located at chromosomal region 11q13.
- Most carcinoids associated with multiple endocrine neoplasia type 1 appear to be of foregut origin.
- Neurofibromatosis type 1 is an autosomal dominant genetic disorder caused by alteration of the NF1 gene at chromosome 17q11.
- Carcinoids in patients with neurofibromatosis type 1 appear to arise primarily in the periampullary region.[9]
- A hereditary form of small intestinal cacinoid tumour has been found which is caused by mutation in the IPMK gene leads to higher risk of developing carcinoid tumors in the small intestine.[10]
- The most frequently reported mutated gene in gastrointestinal carcinoids is β-catenin (CTNNB1).[11]
Embryology
- Carcinoid tumors originate from neuroendocrine cells (enterochromaffin or amine precursor uptake and decarboxylase [APUD] cells) which are derived from neural crest cells embrologically.
- Gastrointestinal carcinoids derive from cells that migrate from the neural crest to the foregut, midgut and hindgut.[12]
Associated Conditions
Goblet cell carcinoid is considered to be a hybrid between an exocrine and endocrine tumor derived from crypt cells of the appendix. They behave in a more aggressive manner than do classical appendiceal carcinoids. Spread is usually to regional lymph nodes, peritoneum, and particularly the ovary. They do not produce sufficient hormonal substances to cause the carcinoid or other endocrine syndromes. In fact, they more closely resemble exocrine than endocrine tumors. The term 'crypt cell carcinoma' has been used for them, and though perhaps more accurate than considering them carcinoids, has not been a successful competitor.
Location
Carcinoid tumors are normally found throughout the gastrointestinal tract from mouth to anus, with the highest concentration of cells in the appendix and small intestine. The pancreas contains a large number of these cells, the biliary tree only a few and the liver normally contains none. Fibrotic lesions are found on endocardium, particularly on the right side of the heart.
Gross Pathology
Gastrointestinal Carcinoid
In the gastric or intestinal wall, carcinoids may occur as firm white, yellow, or gray nodules and may be intramural masses or may protrude into the lumen as polypoid nodules. The overlying gastric or intestinal mucosa may be intact or have focal ulceration.
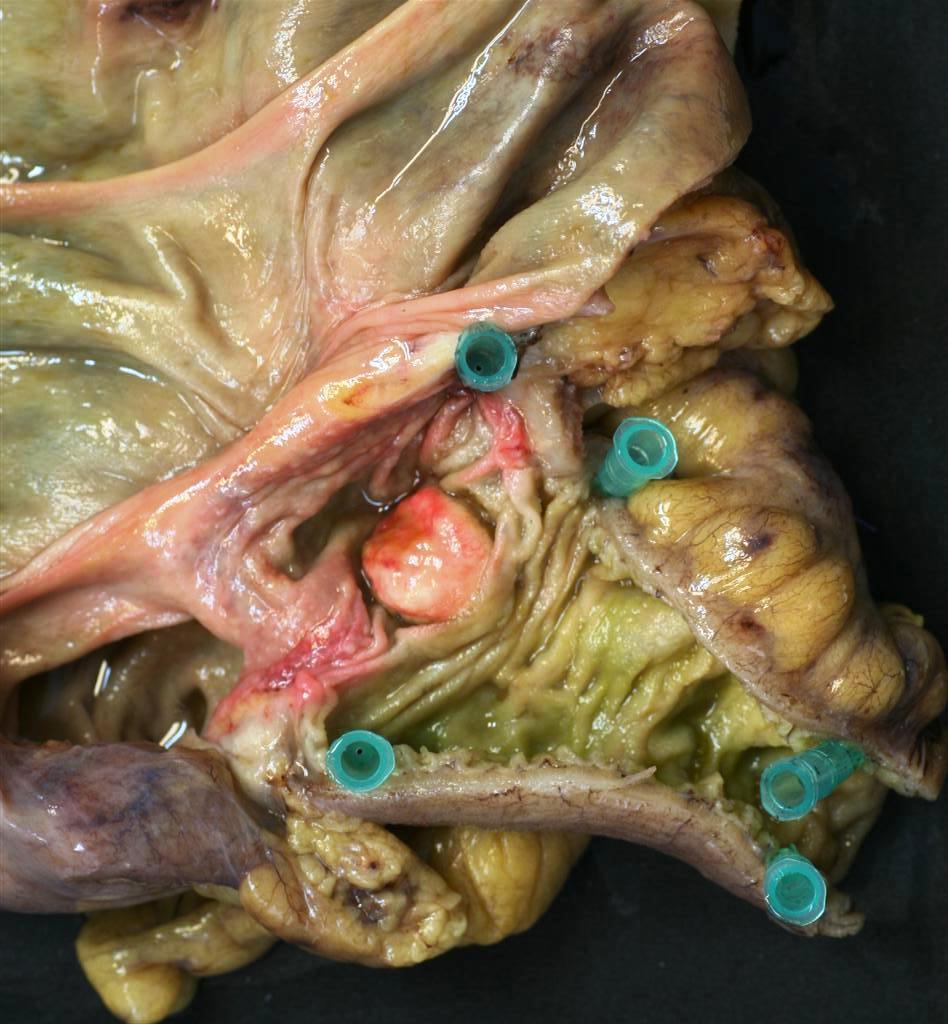 |
Ovarian Carcinoid
Lesions can markedly vary in size. Metastatic carcinoids are nearly always bilateral with scattered tumor deposits present throughout both ovaries. Primary carcinoids of the ovary are invariably unilateral. They form a solid nodule within a cystic teratoma, or form a pure solid hypervascular mass. They can be indistinguishable from other solid neoplasms of the ovary. Lesions can markedly vary in size. Metastatic carcinoids are nearly always bilateral with scattered tumor deposits present throughout both ovaries.[14]
Microscopic Pathology
Goblet Cell Carcinoid of Appendix
Histologically, globet cell carcinoid forms clusters of goblet cells containing mucin with a minor admixture of paneth cells and endocrine cells. The growth pattern is distinctive, typically producing a concentric band of tumor nests interspersed among the muscle and stroma of the appendiceal wall extending up the shaft of the appendix. This makes the lesion difficult to suspect grossly and difficult to measure. Small tumor nests may be camouflaged amongst the muscle or in periappendiceal fat, cytokeratin preparations best demonstrate the tumor cells, mucin stains are also helpful in identifying them.[15]
Gastric or Intestinal Carcinoid
Neuroendocrine cells have uniform nuclei and abundant granular or faintly staining (clear) cytoplasm, and are present as solid or small trabecular clusters, or are dispersed among other cells, which may make them difficult to recognize in sections stained with hematoxylin and eosin, immunostaining enables their exact identification. At the ultrastructural level, neuroendocrine cells contain cytoplasmic membrane-bound dense-cored secretory granules (diameter >80 nm) and may also contain small clear vesicles (diameter 40–80 nm) that correspond to the synaptic vesicles of neurons.[8]
Hepatic Carcinoid
Histologically, the tumor has features of classic carcinoid (i.e. trabecular and pseudoglandular pattern) and dense core granules demonstrated by electron microscopy or by immunohistochemistry (i.e. positive staining by chromogranin antibody).[16]
Lung Carcinoid
- Nests of cells
- Stippled chromatin
- Moderate cytoplasm
- No necrosis
- Low mitotic rate
-
Lung carcinoid low magnitude[17]
-
Lung carcinoid intermediate magnitude[17]
-
Lung carcinoid very high magnitude[17]
-
Lung carcinoid high magnitude[17]
Video
{{#ev:youtube|hTSHJBAD3C4}}
References
- ↑ Kvols LK, Moertel CG, O'Connell MJ, Schutt AJ, Rubin J, Hahn RG (September 1986). "Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue". N. Engl. J. Med. 315 (11): 663–6. doi:10.1056/NEJM198609113151102. PMID 2427948.
- ↑ Launay JM, Birraux G, Bondoux D, Callebert J, Choi DS, Loric S, Maroteaux L (February 1996). "Ras involvement in signal transduction by the serotonin 5-HT2B receptor". J. Biol. Chem. 271 (6): 3141–7. PMID 8621713.
- ↑ Jaffré F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L (January 2009). "Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy". Circ. Res. 104 (1): 113–23. doi:10.1161/CIRCRESAHA.108.180976. PMID 19023134.
- ↑ Xu J, Jian B, Chu R, Lu Z, Li Q, Dunlop J, Rosenzweig-Lipson S, McGonigle P, Levy RJ, Liang B (December 2002). "Serotonin mechanisms in heart valve disease II: the 5-HT2 receptor and its signaling pathway in aortic valve interstitial cells". Am. J. Pathol. 161 (6): 2209–18. doi:10.1016/S0002-9440(10)64497-5. PMID 12466135.
- ↑ Carcinoid cardiac lesions. Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia. http://radiopaedia.org/articles/carcinoid-cardiac-lesions
- ↑ Luis SA, Pellikka PA (January 2016). "Carcinoid heart disease: Diagnosis and management". Best Pract. Res. Clin. Endocrinol. Metab. 30 (1): 149–58. doi:10.1016/j.beem.2015.09.005. PMID 26971851.
- ↑ Druce MR, Bharwani N, Akker SA, Drake WM, Rockall A, Grossman AB (March 2010). "Intra-abdominal fibrosis in a recent cohort of patients with neuroendocrine ('carcinoid') tumours of the small bowel". QJM. 103 (3): 177–85. doi:10.1093/qjmed/hcp191. PMID 20123681.
- ↑ 8.0 8.1 General Information About Gastrointestinal (GI) Carcinoid Tumors.<ref name="pmid2886072">Duh QY, Hybarger CP, Geist R, Gamsu G, Goodman PC, Gooding GA, Clark OH (July 1987). "Carcinoids associated with multiple endocrine neoplasia syndromes". Am. J. Surg. 154 (1): 142–8. PMID 2886072.
- ↑ Karatzas G, Kouraklis G, Karayiannakis A, Patapis P, Givalos N, Kaperonis E (June 2000). "Ampullary carcinoid and jejunal stromal tumour associated with von Recklinghausen's disease presenting as gastrointestinal bleeding and jaundice". Eur J Surg Oncol. 26 (4): 428–9. doi:10.1053/ejso.1999.0911. PMID 10873367.
- ↑ Sei Y, Zhao X, Forbes J, Szymczak S, Li Q, Trivedi A, Voellinger M, Joy G, Feng J, Whatley M, Jones MS, Harper UL, Marx SJ, Venkatesan AM, Chandrasekharappa SC, Raffeld M, Quezado MM, Louie A, Chen CC, Lim RM, Agarwala R, Schäffer AA, Hughes MS, Bailey-Wilson JE, Wank SA (July 2015). "A Hereditary Form of Small Intestinal Carcinoid Associated With a Germline Mutation in Inositol Polyphosphate Multikinase". Gastroenterology. 149 (1): 67–78. doi:10.1053/j.gastro.2015.04.008. PMC 4858647. PMID 25865046.
- ↑ Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T (September 2001). "Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin gene in gastrointestinal carcinoid tumor". Cancer Res. 61 (18): 6656–9. PMID 11559529.
- ↑ Reznek RH (2006). "CT/MRI of neuroendocrine tumours". Cancer Imaging. 6: S163–77. doi:10.1102/1470-7330.2006.9037. PMC 1805060. PMID 17114072.
- ↑ Image courtesy of Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia (original file [1]). [http://radiopaedia.org/licence Creative Commons BY-SA-NC
- ↑ Ovarian carcinoid tumours. Dr Aditya Shetty and Dr Yuranga Weerakkody et al. Radiopaedia 2015. http://radiopaedia.org/articles/ovarian-carcinoid-tumours
- ↑ https://en.wikipedia.org/wiki/Carcinoid
- ↑ Hepatic carcinoid. Dr Henry Knipe and Dr Yuranga Weerakkody et al. Radiopaedia 2015. http://radiopaedia.org/articles/bronchial-carcinoid-tumour
- ↑ 17.0 17.1 17.2 17.3 Typical carcinoid lung tumour. Libre Pathology. http://librepathology.org/wiki/index.php/Typical_carcinoid_lung_tumour Accessed on September, 30 2015
![Lung carcinoid low magnitude[17]](/images/c/c0/Lung_carcinoid_low_mag.jpg)
![Lung carcinoid intermediate magnitude[17]](/images/c/c3/Lung_carcinoid_intermed_mag.jpg)
![Lung carcinoid very high magnitude[17]](/images/8/8a/Lung_carcinoid_very_high_mag.jpg)
![Lung carcinoid high magnitude[17]](/images/2/25/800px-Lung_carcinoid_-_high_mag.jpg)