Captopril: Difference between revisions
Amr Marawan (talk | contribs) |
Amr Marawan (talk | contribs) No edit summary |
||
| Line 25: | Line 25: | ||
'''''For patient information about Captopril, click [[Captopril (patient information)|here]].''''' | '''''For patient information about Captopril, click [[Captopril (patient information)|here]].''''' | ||
{{SB}} Capoten | |||
==Overview== | |||
'''Captopril''' ([[International Nonproprietary Name|rINN]]) ([[International Phonetic Alphabet|IPA]]: {{IPA|[ˈkæptəprɪl]}}) is an [[angiotensin-converting enzyme]] inhibitor ([[ACE inhibitor]]) used for the treatment of [[hypertension]] and some types of [[congestive heart failure]]. Captopril was the first ACE inhibitor developed and was considered a breakthrough both because of its novel mechanism of action and also because of the revolutionary development process. Captopril is commonly marketed by [[Bristol-Myers Squibb]] under the trade name '''Capoten'''. | '''Captopril''' ([[International Nonproprietary Name|rINN]]) ([[International Phonetic Alphabet|IPA]]: {{IPA|[ˈkæptəprɪl]}}) is an [[angiotensin-converting enzyme]] inhibitor ([[ACE inhibitor]]) used for the treatment of [[hypertension]] and some types of [[congestive heart failure]]. Captopril was the first ACE inhibitor developed and was considered a breakthrough both because of its novel mechanism of action and also because of the revolutionary development process. Captopril is commonly marketed by [[Bristol-Myers Squibb]] under the trade name '''Capoten'''. | ||
Revision as of 22:46, 11 February 2014
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 70–75% |
| Metabolism | hepatic |
| Elimination half-life | 1.9 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C9H15NO3S |
| Molar mass | 217.29 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [2]
For patient information about Captopril, click here.
Synonyms / Brand Names: Capoten
Overview
Captopril (rINN) (IPA: Template:IPA) is an angiotensin-converting enzyme inhibitor (ACE inhibitor) used for the treatment of hypertension and some types of congestive heart failure. Captopril was the first ACE inhibitor developed and was considered a breakthrough both because of its novel mechanism of action and also because of the revolutionary development process. Captopril is commonly marketed by Bristol-Myers Squibb under the trade name Capoten.
Category
Antihypertensive Agents, ACE Inhibitors.
FDA Package Insert
CAPTOPRIL (captopril ) tablet
Indications and Usage | Dosage and Administration | Dosage Forms and Strengths | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
 |
Clinical Use
Captopril's main uses are based on its vasodilatation and inhibition of renal function activities. These benefits are most clearly seen in the following conditions:
1) Hypertension
2) Cardiac conditions such as post myocardial infarction and congestive heart failure
3) Preservation of kidney function in diabetic nephropathy
History
Captopril was developed in 1975 by three researchers at the U.S. drug company Squibb (now Bristol-Myers Squibb): Miguel Ondetti, Bernard Rubin and David Cushman. Squibb filed for U.S. patent protection on the drug in February 1976 and U.S. Patent 4,046,889 was granted in September 1977.
The development of captopril was amongst the earliest successes of the revolutionary concept of structure-based drug design. The renin-angiontensin-aldosterone system had been extensively studied in the mid-20th century and it had been decided that this system presented several opportune targets in the development of novel treatments for hypertension. The first two targets that were attempted were renin and ACE. Captopril was the culmination of efforts by Squibb's laboratories to develop an ACE inhibitor.
Ondetti, Cushman and colleagues built on work that had been done in the 1960s by the British pharmacologist John Vane when he was a researcher at the Royal College of Surgeons of England. Working with a Brazilian colleague, Sérgio Ferreira, Vane discovered a peptide in pit viper (Bothrops jararaca) venom which was a 'collected-product inhibitor' of angiotensin II. Captopril was developed from this peptide after it was found via QSAR-based modification that the terminal sulfhydryl moiety of the peptide provided a high potency of ACE inhibition.
Captopril gained FDA approval in June 1981. The drug went generic in the U.S. in February 1996 as a result of the end of market exclusivity for Bristol-Myers Squibb.
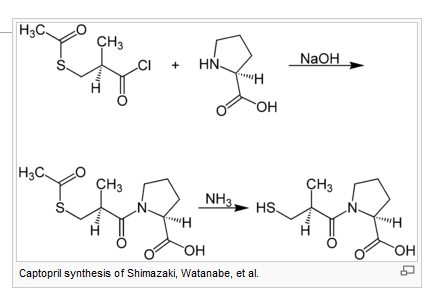
Chemical synthesis
A chemical synthesis of captopril by treatment of L-proline with (2S)-3-acetylthio-2-methylpropanoyl chloride under basic conditions (NaOH), followed by aminolysis of the protective acetyl group to unmask the drug's free thiol, is depicted in the figure at right.
Developments from captopril
Limitations of captopril
The adverse drug reaction (ADR) profile of captopril is similar to other ACE inhibitors, with cough being the most common ADR (Rossi, 2006). However, captopril is also commonly associated with rash and taste disturbances (metallic or loss of taste), which are attributed to the unique sulfhydryl moiety (Atkinson & Robertson, 1979).
Captopril also has a relatively poor pharmacokinetic profile. The short half-life necessitates 2–3 times daily dosing, which may reduce patient compliance.
Subsequent ACE inhibitors
The adverse effect and pharmacokinetic limitations of captopril stimulated the development enalapril and subsequent ACE inhibitors. These were specifically designed to lack the sulfhydryl moiety believed to be responsible for rash and taste disturbance (Patchett et al., 1980). Most subsequent ACE inhibitors are given as prodrugs, to improve oral bioavailability. All have a longer half-life and are given once daily, which may improve patient compliance.
References
- Atkinson AB, Robertson JIS. Captopril in the treatment of hypertension and cardiac failure. Lancet 1979;2(8147):836–9. PMID 90928
- Patchett AA, Harris E, Tristam EQ, et al. A new class of angiotensin-converting enzyme inhibitors. Nature 1980;288(5788):280–3. PMID 6253826
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- Smith CG, Vane JR. The discovery of captopril. FASEB J 2003;17:788-9. Fulltext. PMID 12724335.
See also
External links
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Cardiovascular Drugs
- Drugs
- ACE inhibitors