Capecitabine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: Capecitabine-WARFARIN INTERACTION
See full prescribing information for complete Boxed Warning.
|
Overview
Capecitabine is an antimetabolite that is FDA approved for the treatment of adjuvant colon cancer, metastatic colorectal cancer, metastatic breast cancer. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, hand-and-foot syndrome, nausea, vomiting, abdominal pain, fatigue/weakness, and hyperbilirubinemia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Colorectal Cancer
- Capecitabine tablets are indicated as a single agent for adjuvant treatment in patients with Dukes’ C colon cancer who have undergone complete resection of the primary tumor when treatment with fluoropyrimidine therapy alone is preferred. Capecitabine was non-inferior to 5-fluorouracil and leucovorin (5-FU/LV) for disease-free survival (DFS). Physicians should consider results of combination chemotherapy trials, which have shown improvement in DFS and OS, when prescribing single-agent capecitabine in the adjuvant treatment of Dukes’ C colon cancer.
- Capecitabine tablets are indicated as first-line treatment of patients with metastatic colorectal carcinoma when treatment with fluoropyrimidine therapy alone is preferred. Combination chemotherapy has shown a survival benefit compared to 5-FU/LV alone. A survival benefit over 5-FU/LV has not been demonstrated with capecitabine monotherapy. Use of capecitabine instead of 5-FU/LV in combinations has not been adequately studied to assure safety or preservation of the survival advantage.
Breast Cancer
- Capecitabine tablets in combination with docetaxel are indicated for the treatment of patients with metastatic breast cancer after failure of prior anthracycline-containing chemotherapy.
- Capecitabine tablets monotherapy is also indicated for the treatment of patients with metastatic breast cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen or resistant to paclitaxel and for whom further anthracycline therapy is not indicated (e.g., patients who have received cumulative doses of 400 mg/m2 of doxorubicin or doxorubicin equivalents). Resistance is defined as progressive disease while on treatment, with or without an initial response or relapse within 6 months of completing treatment with an anthracycline-containing adjuvant regimen.
Standard Starting Dose
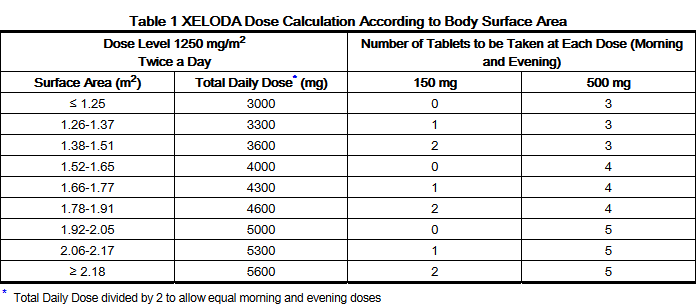
- The recommended dose of capecitabine is 1250 mg/m2 administered orally twice daily (morning and evening; equivalent to 2500 mg/m2 total daily dose) for 2 weeks followed by a 1 week rest period given as 3 week cycles (see Table 1).
- Adjuvant treatment in patients with Dukes’ C colon cancer is recommended for a total of 6 months i.e., capecitabine 1250 mg/m2 orally twice daily for 2 weeks followed by a 1 week rest period, given as 3 week cycles for a total of 8 cycles (24 weeks).
- In Combination With Docetaxel (Metastatic Breast Cancer)
- In combination with docetaxel, the recommended dose of capecitabine is 1250 mg/m2 twice daily for 2 weeks followed by a 1 week rest period, combined with docetaxel at 75 mg/m2 as a 1 hour intravenous infusion every 3 weeks. Pre-medication, according to the docetaxel labeling, should be started prior to docetaxel administration for patients receiving the capecitabine tablets plus docetaxel combination. Table 1 displays the total daily dose of capecitabine by body surface area and the number of tablets to be taken at each dose.
Dose Management Guidelines
- General
- Capecitabine dosage may need to be individualized to optimize patient management. Patients should be carefully monitored for toxicity and doses of capecitabine should be modified as necessary to accommodate individual patient tolerance to treatment. Toxicity due to capecitabine tablet administration may be managed by symptomatic treatment, dose interruptions and adjustment of capecitabine dose. Once the dose has been reduced, it should not be increased at a later time. Doses of capecitabine omitted for toxicity are not replaced or restored; instead the patient should resume the planned treatment cycles.
- The dose of phenytoin and the dose of coumarin-derivative anticoagulants may need to be reduced when either drug is administered concomitantly with capecitabine tablets.
- Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast Cancer)
- Capecitabine dose modification scheme as described below (see Table 2) is recommended for the management of adverse reactions.
- In Combination with Docetaxel (Metastatic Breast Cancer)
- Dose modifications of capecitabine tablets for toxicity should be made according to Table 2 above for capecitabine. At the beginning of a treatment cycle, if a treatment delay is indicated for either capecitabine or docetaxel, then administration of both agents should be delayed until the requirements for restarting both drugs are met.
- The dose reduction schedule for docetaxel when used in combination with capecitabine for the treatment of metastatic breast cancer is shown in Table 3.
Adjustment of Starting Dose in Special Populations
- Renal Impairment
- No adjustment to the starting dose of capecitabine is recommended in patients with mild renal impairment (creatinine clearance = 51 to 80 mL/min). In patients with moderate renal impairment (baseline creatinine clearance = 30 to 50 mL/min), a dose reduction to 75% of the capecitabine starting dose when used as monotherapy or in combination with docetaxel (from 1250 mg/m2 to 950 mg/m2 twice daily) is recommended. Subsequent dose adjustment is recommended as outlined in Table 2 and Table 3 (depending on the regimen) if a patient develops a grade 2 to 4 adverse event. The starting dose adjustment recommendations for patients with moderate renal impairment apply to both capecitabine monotherapy and capecitabine in combination use with docetaxel.
- Geriatrics
- Physicians should exercise caution in monitoring the effects of capecitabine tablets in the elderly. Insufficient data are available to provide a dosage recommendation.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Metastatic colorectal cancer, First-line therapy, in combination with bevacizumab and oxaliplatin
- Dosing Information
- Oral capecitabine 1000 mg/m(2) twice daily days 1 to 14) repeated every 3 weeks.[1]
Metastatic breast cancer, In combination with bevacizumab in patients previously treated with an anthracycline and a taxane
- Dosing Information
- Oral capecitabine 2500 mg/m(2)/day given twice daily for 14 days.[2]
Non–Guideline-Supported Use
Colon cancer, Adjuvant therapy, stage III, in combination with oxaliplatin
- Dosing Information
- Capecitabine 1000 mg/m(2) orally twice daily on days 1 to 14 of a 3-week cycle for a total of 8 cycles.[3]
Colorectal cancer, Nonresectable, advanced, or metastatic; first-line in combination with oxaliplatin
- Dosing Information
- Oral capecitabine 1000 mg/m(2) twice daily for 2 weeks (wk) of a 3-wk cycle.[4]
Esophagogastric cancer, Advanced or metastatic, in combination with chemotherapeutic agents
- Dosing Information
- Capecitabine 1000 mg/m(2) orally twice daily for 14 days.[5]
Esophagogastric cancer, First-line therapy for advanced or metastatic disease, in combination with epirubicin and oxaliplatin or cisplatin
- Dosing Information
- Oral capecitabine 625 mg/m(2) twice daily.[6]
Pancreatic cancer, Monotherapy
- Dosing Information
- 1250 (mg/m(2) of oral capecitabine twice daily in 3-week cycles.
Pancreatic cancer, Locally advanced or metastatic, first-line therapy in combination with gemcitabine
- Dosing Information
- Capecitabine 830 mg/m(2) orally twice daily for 3 weeks.[7]
Ovarian cancer
- Dosing Information
- Oral capecitabine 2500 mg/m(2)/day in 2 divided doses for 14 days on and 7 days off of a 21-day cycle.[8]
Metastatic renal cell carcinoma
- Dosing Information
- Capecitabine 1250 mg/m(2) twice daily for 14 days, followed by 1 week of rest every 3 weeks.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Capecitabine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Capecitabine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Capecitabine in pediatric patients.
Contraindications
- Dihydropyrimidine Dehydrogenase (DPD) Deficiency
- Capecitabine is contraindicated in patients with known dihydropyrimidine dehydrogenase (DPD) deficiency.
- Severe Renal Impairment
- Capecitabine is contraindicated in patients with severe renal impairment (creatinine clearance below 30 mL/min).
- Hypersensitivity
- Capecitabine is contraindicated in patients with known hypersensitivity to capecitabine or to any of its components. Capecitabine is contraindicated in patients who have a known hypersensitivity to 5-fluorouracil.
Warnings
|
WARNING: Capecitabine-WARFARIN INTERACTION
See full prescribing information for complete Boxed Warning.
|
Precautions
- Diarrhea
- Capecitabine can induce diarrhea, sometimes severe. Patients with severe diarrhea should be carefully monitored and given fluid and electrolyte replacement if they become dehydrated. In 875 patients with either metastatic breast or colorectal cancer who received capecitabine monotherapy, the median time to first occurrence of grade 2 to 4 diarrhea was 34 days (range from 1 to 369 days). The median duration of grade 3 to 4 diarrhea was 5 days. National Cancer Institute of Canada (NCIC) grade 2 diarrhea is defined as an increase of 4 to 6 stools/day or nocturnal stools, grade 3 diarrhea as an increase of 7 to 9 stools/day or incontinence and malabsorption and grade 4 diarrhea as an increase of ≥ 10 stools/day or grossly bloody diarrhea or the need for parenteral support. If grade 2, 3 or 4 diarrhea occurs, administration of capecitabine should be immediately interrupted until the diarrhea resolves or decreases in intensity to grade 1. Following a reoccurrence of grade 2 diarrhea or occurrence of any grade 3 or 4 diarrhea, subsequent doses of capecitabine should be decreased. Standard antidiarrheal treatments (e.g., loperamide) are recommended.
- Necrotizing enterocolitis (typhlitis) has been reported.
- Coagulopathy
- Patients receiving concomitant capecitabine and oral coumarin-derivative anticoagulant therapy should have their anticoagulant response (INR or prothrombin time) monitored closely with great frequency and the anticoagulant dose should be adjusted accordingly.
- Cardiotoxicity
- The cardiotoxicity observed with capecitabine includes myocardial infarction/ischemia, angina, dysrhythmias, cardiac arrest, cardiac failure, sudden death, electrocardiographic changes and cardiomyopathy. These adverse reactions may be more common in patients with a prior history of coronary artery disease.
- Dihydropyrimidine Dehydrogenase Deficiency
- Rarely, unexpected, severe toxicity (e.g., stomatitis, diarrhea, neutropenia and neurotoxicity) associated with 5-fluorouracil has been attributed to a deficiency of dihydropyrimidine dehydrogenase (DPD) activity. A link between decreased levels of DPD and increased, potentially fatal toxic effects of 5-fluorouracil therefore cannot be excluded.
- Renal Insufficiency
- Patients with moderate renal impairment at baseline require dose reduction. Patients with mild and moderate renal impairment at baseline should be carefully monitored for adverse reactions. Prompt interruption of therapy with subsequent dose adjustments is recommended if a patient develops a grade 2 to 4 adverse event as outlined in Table 2.
- Pregnancy
- Capecitabine may cause fetal harm when given to a pregnant woman. Capecitabine caused embryolethality and teratogenicity in mice and embryolethality in monkeys when administered during organogenesis. If this drug is used during pregnancy or if a patient becomes pregnant while receiving capecitabine, the patient should be apprised of the potential hazard to the fetus.
- Hand-and-Foot Syndrome
- Hand-and-foot syndrome (palmar-plantar erythrodysesthesia or chemotherapy-induced acral erythema) is a cutaneous toxicity. Median time to onset was 79 days (range from 11 to 360 days) with a severity range of grades 1 to 3 for patients receiving capecitabine monotherapy in the metastatic setting. Grade 1 is characterized by any of the following: numbness, dysesthesia/paresthesia, tingling, painless swelling or erythema of the hands and/or feet and/or discomfort which does not disrupt normal activities. Grade 2 hand-and-foot syndrome is defined as painful erythema and swelling of the hands and/or feet and/or discomfort affecting the patient’s activities of daily living. Grade 3 hand-and-foot syndrome is defined as moist desquamation, ulceration, blistering or severe pain of the hands and/or feet and/or severe discomfort that causes the patient to be unable to work or perform activities of daily living. If grade 2 or 3 hand-and-foot syndrome occurs, administration of capecitabine should be interrupted until the event resolves or decreases in intensity to grade 1. Following grade 3 hand-and-foot syndrome, subsequent doses of capecitabine should be decreased.
- Hyperbilirubinemia
- In 875 patients with either metastatic breast or colorectal cancer who received at least one dose of capecitabine 1250 mg/m2 twice daily as monotherapy for 2 weeks followed by a 1 week rest period, grade 3 (1.5 to 3 x ULN) hyperbilirubinemia occurred in 15.2% (n = 133) of patients and grade 4 (> 3 x ULN) hyperbilirubinemia occurred in 3.9% (n = 34) of patients. Of 566 patients who had hepatic metastases at baseline and 309 patients without hepatic metastases at baseline, grade 3 or 4 hyperbilirubinemia occurred in 22.8% and 12.3%, respectively. Of the 167 patients with grade 3 or 4 hyperbilirubinemia, 18.6% (n = 31) also had post-baseline elevations (grades 1 to 4, without elevations at baseline) in alkaline phosphatase and 27.5% (n = 46) had post-baseline elevations in transaminases at any time (not necessarily concurrent). The majority of these patients, 64.5% (n = 20) and 71.7% (n = 33), had liver metastases at baseline. In addition, 57.5% (n = 96) and 35.3% (n = 59) of the 167 patients had elevations (grades 1 to 4) at both pre-baseline and post-baseline in alkaline phosphatase or transaminases, respectively. Only 7.8% (n = 13) and 3% (n = 5) had grade 3 or 4 elevations in alkaline phosphatase or transaminases.
- In the 596 patients treated with capecitabine as first-line therapy for metastatic colorectal cancer, the incidence of grade 3 or 4 hyperbilirubinemia was similar to the overall clinical trial safety database of capecitabine monotherapy. The median time to onset for grade 3 or 4 hyperbilirubinemia in the colorectal cancer population was 64 days and median total bilirubin increased from 8 μm/L at baseline to 13 μm/L during treatment with capecitabine. Of the 136 colorectal cancer patients with grade 3 or 4 hyperbilirubinemia, 49 patients had grade 3 or 4 hyperbilirubinemia as their last measured value, of which 46 had liver metastases at baseline.
- In 251 patients with metastatic breast cancer who received a combination of capecitabine and docetaxel, grade 3 (1.5 to 3 x ULN) hyperbilirubinemia occurred in 7% (n = 17) and grade 4 (> 3 x ULN) hyperbilirubinemia occurred in 2% (n = 5).
- If drug-related grade 3 to 4 elevations in bilirubin occur, administration of capecitabine should be immediately interrupted until the hyperbilirubinemia decreases to ≤ 3 x ULN.
- Hematologic
- In 875 patients with either metastatic breast or colorectal cancer who received a dose of 1250 mg/m2 administered twice daily as monotherapy for 2 weeks followed by a 1 week rest period, 3.2%, 1.7% and 2.4% of patients had grade 3 or 4 neutropenia, thrombocytopenia or decreases in hemoglobin, respectively. In 251 patients with metastatic breast cancer who received a dose of capecitabine in combination with docetaxel, 68% had grade 3 or 4 neutropenia, 2.8% had grade 3 or 4 thrombocytopenia and 9.6% had grade 3 or 4 anemia.
- Patients with baseline neutrophil counts of < 1.5 x 109/L and/or thrombocyte counts of < 100 x 109/L should not be treated with capecitabine. If unscheduled laboratory assessments during a treatment cycle show grade 3 or 4 hematologic toxicity, treatment with capecitabine should be interrupted.
- Geriatric Patients
- Patients ≥ 80 years old may experience a greater incidence of grade 3 or 4 adverse reactions. In 875 patients with either metastatic breast or colorectal cancer who received capecitabine monotherapy, 62% of the 21 patients ≥ 80 years of age treated with capecitabine experienced a treatment-related grade 3 or 4 adverse event: diarrhea in six (28.6%), nausea in three (14.3%), hand-foot syndrome in three (14.3%) and vomiting in two (9.5%) patients. Among the ten patients 70 years of age and greater (no patients were > 80 years of age) treated with capecitabine in combination with docetaxel, 30% (3 out of 10) of patients experienced grade 3 or 4 diarrhea and stomatitis and 40% (4 out of 10) experienced grade 3 hand-and-foot syndrome.
- Among the 67 patients ≥ 60 years of age receiving capecitabine in combination with docetaxel, the incidence of grade 3 or 4 treatment-related adverse reactions, treatment-related serious adverse reactions, withdrawals due to adverse reactions, treatment discontinuations due to adverse reactions and treatment discontinuations within the first two treatment cycles was higher than in the < 60 years of age patient group.
- In 995 patients receiving capecitabine as adjuvant therapy for Dukes’ C colon cancer after resection of the primary tumor, 41% of the 398 patients ≥ 65 years of age treated with capecitabine experienced a treatment-related grade 3 or 4 adverse event: hand-foot syndrome in 75 (18.8%), diarrhea in 52 (13.1%), stomatitis in 12 (3%), neutropenia/granulocytopenia in 11 (2.8%), vomiting in six (1.5%) and nausea in five (1.3%) patients. In patients ≥ 65 years of age (all randomized population; capecitabine 188 patients, 5-FU/LV 208 patients) treated for Dukes’ C colon cancer after resection of the primary tumor, the hazard ratios for disease-free survival and overall survival for capecitabine compared to 5-FU/LV were 1.01 (95% C.I. 0.80 to 1.27) and 1.04 (95% C.I. 0.79 to 1.37), respectively.
- Hepatic Insufficiency
- Patients with mild to moderate hepatic dysfunction due to liver metastases should be carefully monitored when capecitabine is administered. The effect of severe hepatic dysfunction on the disposition of capecitabine is not known.
- Combination with Other Drugs
- Use of capecitabine in combination with irinotecan has not been adequately studied.
Adverse Reactions
Clinical Trials Experience
- Table 4 shows the adverse reactions occurring in ≥ 5% of patients from one phase 3 trial in patients with Dukes’ C colon cancer who received at least one dose of study medication and had at least one safety assessment. A total of 995 patients were treated with 1250 mg/m2 twice a day of capecitabine administered for 2 weeks followed by a 1 week rest period and 974 patients were administered 5-FU and leucovorin (20 mg/m2 leucovorin IV followed by 425 mg/m2 IV bolus 5-FU on days 1 to 5 every 28 days). The median duration of treatment was 164 days for capecitabine-treated patients and 145 days for 5-FU/LV-treated patients. A total of 112 (11%) and 73 (7%) capecitabine and 5-FU/LV-treated patients, respectively, discontinued treatment because of adverse reactions. A total of 18 deaths due to all causes occurred either on study or within 28 days of receiving study drug: 8 (0.8%) patients randomized to capecitabine and 10 (1%) randomized to 5-FU/LV.
- Table 5 shows grade 3/4 laboratory abnormalities occurring in ≥ 1% of patients from one phase 3 trial in patients with Dukes’ C colon cancer who received at least one dose of study medication and had at least one safety assessment.
- Monotherapy
- Table 6 shows the adverse reactions occurring in ≥ 5% of patients from pooling the two phase 3 trials in first line metastatic colorectal cancer. A total of 596 patients with metastatic colorectal cancer were treated with 1250 mg/m2 twice a day of capecitabine administered for 2 weeks followed by a 1 week rest period and 593 patients were administered 5-FU and leucovorin in the Mayo regimen (20 mg/m2 leucovorin IV followed by 425 mg/m2 IV bolus 5-FU, on days 1 to 5, every 28 days). In the pooled colorectal database the median duration of treatment was 139 days for capecitabine-treated patients and 140 days for 5-FU/LV-treated patients. A total of 78 (13%) and 63 (11%) capecitabine and 5-FU/LV-treated patients, respectively, discontinued treatment because of adverse reactions/intercurrent illness. A total of 82 deaths due to all causes occurred either on study or within 28 days of receiving study drug: 50 (8.4%) patients randomized to capecitabine and 32 (5.4%) randomized to 5-FU/LV.
- In Combination with Docetaxel
- The following data are shown for the combination study with capecitabine and docetaxel in patients with metastatic breast cancer in Table 7 and Table 8. In the capecitabine and docetaxel combination arm the treatment was capecitabine administered orally 1250 mg/m2 twice daily as intermittent therapy (2 weeks of treatment followed by 1 week without treatment) for at least 6 weeks and docetaxel administered as a 1 hour intravenous infusion at a dose of 75 mg/m2 on the first day of each 3 week cycle for at least 6 weeks. In the monotherapy arm docetaxel was administered as a 1 hour intravenous infusion at a dose of 100 mg/m2 on the first day of each 3 week cycle for at least 6 weeks. The mean duration of treatment was 129 days in the combination arm and 98 days in the monotherapy arm. A total of 66 patients (26%) in the combination arm and 49 (19%) in the monotherapy arm withdrew from the study because of adverse reactions. The percentage of patients requiring dose reductions due to adverse reactions was 65% in the combination arm and 36% in the monotherapy arm. The percentage of patients requiring treatment interruptions due to adverse reactions in the combination arm was 79%. Treatment interruptions were part of the dose modification scheme for the combination therapy arm but not for the docetaxel monotherapy-treated patients.
- Monotherapy
- The following data are shown for the study in stage IV breast cancer patients who received a dose of 1250 mg/m2 administered twice daily for 2 weeks followed by a 1 week rest period. The mean duration of treatment was 114 days. A total of 13 out of 162 patients (8%) discontinued treatment because of adverse reactions/intercurrent illness.
Clinically Relevant Adverse Events in < 5% of Patients
- Monotherapy (Metastatic Colorectal Cancer, Adjuvant Colorectal Cancer, Metastatic Breast Cancer)
- Capecitabine In Combination with Docetaxel (Metastatic Breast Cancer)
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Capecitabine in the drug label.
Drug Interactions
- Anticoagulants
- Altered coagulation parameters and/or bleeding have been reported in patients taking Capecitabine concomitantly with coumarin-derivative anticoagulants such as warfarin and phenprocoumon. These events occurred within several days and up to several months after initiating Capecitabine therapy and, in a few cases, within 1 month after stopping Capecitabine. These events occurred in patients with and without liver metastases. In a drug interaction study with single-dose warfarin administration, there was a significant increase in the mean AUC of S-warfarin. The maximum observed INR value increased by 91%. This interaction is probably due to an inhibition of cytochrome P450 2C9 by capecitabine and/or its metabolites.
- Phenytoin
- The level of phenytoin should be carefully monitored in patients taking Capecitabine and phenytoin dose may need to be reduced. Postmarketing reports indicate that some patients receiving Capecitabine and phenytoin had toxicity associated with elevated phenytoin levels. Formal drug-drug interaction studies with phenytoin have not been conducted, but the mechanism of interaction is presumed to be inhibition of the CYP2C9 isoenzyme by capecitabine and/or its metabolites.
- Leucovorin
- The concentration of 5-fluorouracil is increased and its toxicity may be enhanced by leucovorin. Deaths from severe enterocolitis, diarrhea, and dehydration have been reported in elderly patients receiving weekly leucovorin and fluorouracil.
- CYP2C9 substrates
- Drug-Food Interaction
- Food was shown to reduce both the rate and extent of absorption of capecitabine. In all clinical trials, patients were instructed to administer Capecitabine within 30 minutes after a meal. It is recommended that Capecitabine be administered with food.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- Capecitabine can cause fetal harm when administered to a pregnant woman. Capecitabine at doses of 198 mg/kg/day during organogenesis caused malformations and embryo death in mice. In separate pharmacokinetic studies, this dose in mice produced 5'-DFUR AUC values about 0.2 times the corresponding values in patients administered the recommended daily dose. Malformations in mice included cleft palate, anophthalmia, microphthalmia, oligodactyly, polydactyly, syndactyly, kinky tail and dilation of cerebral ventricles. At doses of 90 mg/kg/day, capecitabine given to pregnant monkeys during organogenesis caused fetal death. This dose produced 5'-DFUR AUC values about 0.6 times the corresponding values in patients administered the recommended daily dose.
- There are no adequate and well controlled studies of Capecitabine in pregnant women. If this drug is used during pregnancy, or if a patient becomes pregnant while receiving Capecitabine, the patient should be apprised of the potential hazard to the fetus. Women should be advised to avoid becoming pregnant while receiving treatment with Capecitabine.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Capecitabine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Capecitabine during labor and delivery.
Nursing Mothers
- Lactating mice given a single oral dose of capecitabine excreted significant amounts of capecitabine metabolites into the milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from capecitabine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Capecitabine in pediatric patients have not been established. No clinical benefit was demonstrated in two single arm trials in pediatric patients with newly diagnosed brainstem gliomas and high grade gliomas. In both trials, pediatric patients received an investigational pediatric formulation of capecitabine concomitantly with and following completion of radiation therapy (total dose of 5580 cGy in 180 cGy fractions). The relative bioavailability of the investigational formulation to Capecitabine was similar.
- The first trial was conducted in 22 pediatric patients (median age 8 years, range 5-17 years) with newly diagnosed non-disseminated intrinsic diffuse brainstem gliomas and high grade gliomas. In the dose-finding portion of the trial, patients received capecitabine with concomitant radiation therapy at doses ranging from 500 mg/m2 to 850 mg/m2 every 12 hours for up to 9 weeks. After a 2 week break, patients received 1250 mg/m2 capecitabine every 12 hours on Days 1-14 of a 21-day cycle for up to 3 cycles. The maximum tolerated dose (MTD) of capecitabine administered concomitantly with radiation therapy was 650 mg/m2 every 12 hours. The major dose limiting toxicities were palmar-plantar erythrodysesthesia and alanine aminotransferase (ALT) elevation.
- The second trial was conducted in 34 additional pediatric patients with newly diagnosed non-disseminated intrinsic diffuse brainstem gliomas (median age 7 years, range 3-16 years) and 10 pediatric patients who received the MTD of capecitabine in the dose-finding trial and met the eligibility criteria for this trial. All patients received 650 mg/m2 capecitabine every 12 hours with concomitant radiation therapy for up to 9 weeks. After a 2 week break, patients received 1250 mg/m2 capecitabine every 12 hours on Days 1-14 of a 21-day cycle for up to 3 cycles.
- There was no improvement in one-year progression-free survival rate and one-year overall survival rate in pediatric patients with newly diagnosed intrinsic brainstem gliomas who received capecitabine relative to a similar population of pediatric patients who participated in other clinical trials.
- The adverse reaction profile of capecitabine was consistent with the known adverse reaction profile in adults, with the exception of laboratory abnormalities which occurred more commonly in pediatric patients. The most frequently reported laboratory abnormalities (per-patient incidence ≥40%) were increased ALT (75%), lymphocytopenia (73%), leukopenia (73%), hypokalemia (68%), thrombocytopenia (57%), hypoalbuminemia (55%), neutropenia (50%), low hematocrit (50%), hypocalcemia (48%), hypophosphatemia (45%) and hyponatremia (45%).
Geriatic Use
Physicians should pay particular attention to monitoring the adverse effects of Capecitabine in the elderly.
Gender
There is no FDA guidance on the use of Capecitabine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Capecitabine with respect to specific racial populations.
Renal Impairment
- Patients with moderate (creatinine clearance = 30 to 50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment showed higher exposure for capecitabine, 5-FDUR, and FBAL than in those with normal renal function.
Hepatic Impairment
- Exercise caution when patients with mild to moderate hepatic dysfunction due to liver metastases are treated with Capecitabine. The effect of severe hepatic dysfunction on Capecitabine is not known.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Capecitabine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Capecitabine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Capecitabine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Capecitabine in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- The manifestations of acute overdose would include nausea, vomiting, diarrhea, gastrointestinal irritation and bleeding, and bone marrow depression.
- Single doses of Capecitabine were not lethal to mice, rats, and monkeys at doses up to 2000 mg/kg (2.4, 4.8, and 9.6 times the recommended human daily dose on a mg/m2 basis).
Management
- Medical management of overdose should include customary supportive medical interventions aimed at correcting the presenting clinical manifestations. Although no clinical experience using dialysis as a treatment for Capecitabine overdose has been reported, dialysis may be of benefit in reducing circulating concentrations of 5'-DFUR, a low–molecular-weight metabolite of the parent compound.
Chronic Overdose
There is limited information regarding Chronic Overdose of Capecitabine in the drug label.
Pharmacology
Mechanism of Action
- Enzymes convert capecitabine to 5-fluorouracil (5-FU) in vivo. Both normal and tumor cells metabolize 5-FU to 5-fluoro-2'-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). These metabolites cause cell injury by two different mechanisms. First, FdUMP and the folate cofactor, N5-10-methylenetetrahydrofolate, bind to thymidylate synthase (TS) to form a covalently bound ternary complex. This binding inhibits the formation of thymidylate from 2'-deoxyuridylate. Thymidylate is the necessary precursor of thymidine triphosphate, which is essential for the synthesis of DNA, so that a deficiency of this compound can inhibit cell division. Second, nuclear transcriptional enzymes can mistakenly incorporate FUTP in place of uridine triphosphate (UTP) during the synthesis of RNA. This metabolic error can interfere with RNA processing and protein synthesis.
Structure
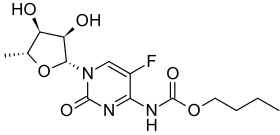
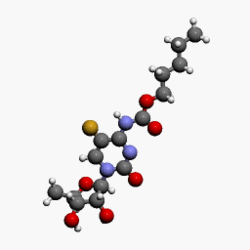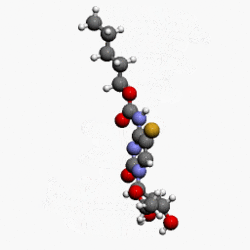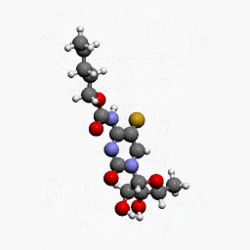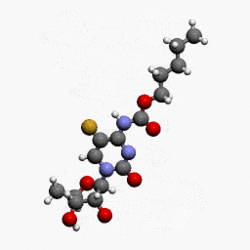
- Capecitabine (capecitabine) is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5'-deoxy-5-fluorouridine (5'-DFUR) which is converted to 5-fluorouracil.
- The chemical name for capecitabine is 5'-deoxy-5-fluoro-N-[(pentyloxy) carbonyl]-cytidine and has a molecular weight of 359.35. Capecitabine has the following structural formula:
- Capecitabine is a white to off-white crystalline powder with an aqueous solubility of 26 mg/mL at 20°C.
- Capecitabine is supplied as biconvex, oblong film-coated tablets for oral administration. Each light peach-colored tablet contains 150 mg capecitabine and each peach-colored tablet contains 500 mg capecitabine. The inactive ingredients in Capecitabine include: anhydrous lactose, croscarmellose sodium, hydroxypropyl methylcellulose, microcrystalline cellulose, magnesium stearate and purified water. The peach or light peach film coating contains hydroxypropyl methylcellulose, talc, titanium dioxide, and synthetic yellow and red iron oxides.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Capecitabine in the drug label.
Pharmacokinetics
- Absorption
- Following oral administration of 1255 mg/m2 BID to cancer patients, capecitabine reached peak blood levels in about 1.5 hours (Tmax) with peak 5-FU levels occurring slightly later, at 2 hours. Food reduced both the rate and extent of absorption of capecitabine with mean Cmax and AUC0-∞ decreased by 60% and 35%, respectively. The Cmax and AUC0-∞ of 5-FU were also reduced by food by 43% and 21%, respectively. Food delayed Tmax of both parent and 5-FU by 1.5 hours.
- The pharmacokinetics of Capecitabine and its metabolites have been evaluated in about 200 cancer patients over a dosage range of 500 to 3500 mg/m2/day. Over this range, the pharmacokinetics of Capecitabine and its metabolite, 5'-DFCR were dose proportional and did not change over time. The increases in the AUCs of 5'-DFUR and 5-FU, however, were greater than proportional to the increase in dose and the AUC of 5-FU was 34% higher on day 14 than on day 1. The interpatient variability in the Cmax and AUC of 5-FU was greater than 85%.
- Distribution
- Plasma protein binding of capecitabine and its metabolites is less than 60% and is not concentration-dependent. Capecitabine was primarily bound to human albumin (approximately 35%). Capecitabine has a low potential for pharmacokinetic interactions related to plasma protein binding.
- Bioactivation and Metabolism
- Capecitabine is extensively metabolized enzymatically to 5-FU. In the liver, a 60 kDa carboxylesterase hydrolyzes much of the compound to 5'-deoxy-5-fluorocytidine (5'-DFCR). Cytidine deaminase, an enzyme found in most tissues, including tumors, subsequently converts 5'-DFCR to 5'-DFUR. The enzyme, thymidine phosphorylase (dThdPase), then hydrolyzes 5'-DFUR to the active drug 5-FU. Many tissues throughout the body express thymidine phosphorylase. Some human carcinomas express this enzyme in higher concentrations than surrounding normal tissues. Following oral administration of Capecitabine 7 days before surgery in patients with colorectal cancer, the median ratio of 5-FU concentration in colorectal tumors to adjacent tissues was 2.9 (range from 0.9 to 8.0). These ratios have not been evaluated in breast cancer patients or compared to 5-FU infusion.
- The enzyme dihydropyrimidine dehydrogenase hydrogenates 5-FU, the product of capecitabine metabolism, to the much less toxic 5-fluoro-5, 6-dihydro-fluorouracil (FUH2). Dihydropyrimidinase cleaves the pyrimidine ring to yield 5-fluoro-ureido-propionic acid (FUPA). Finally, β-ureido-propionase cleaves FUPA to α-fluoro-β-alanine (FBAL) which is cleared in the urine.
- In vitro enzymatic studies with human liver microsomes indicated that capecitabine and its metabolites (5'-DFUR, 5'-DFCR, 5-FU, and FBAL) did not inhibit the metabolism of test substrates by cytochrome P450 isoenzymes 1A2, 2A6, 3A4, 2C19, 2D6, and 2E1.
- Excretion
- Capecitabine and its metabolites are predominantly excreted in urine; 95.5% of administered capecitabine dose is recovered in urine. Fecal excretion is minimal (2.6%). The major metabolite excreted in urine is FBAL which represents 57% of the administered dose. About 3% of the administered dose is excreted in urine as unchanged drug. The elimination half-life of both parent capecitabine and 5-FU was about 0.75 hour.
- Effect of Age, Gender, and Race on the Pharmacokinetics of Capecitabine
- A population analysis of pooled data from the two large controlled studies in patients with metastatic colorectal cancer (n=505) who were administered Capecitabine at 1250 mg/m2 twice a day indicated that gender (202 females and 303 males) and race (455 white/Caucasian patients, 22 black patients, and 28 patients of other race) have no influence on the pharmacokinetics of 5'-DFUR, 5-FU and FBAL. Age has no significant influence on the pharmacokinetics of 5'-DFUR and 5-FU over the range of 27 to 86 years. A 20% increase in age results in a 15% increase in AUC of FBAL.
- Following oral administration of 825 mg/m2 capecitabine twice daily for 14 days, Japanese patients (n=18) had about 36% lower Cmax and 24% lower AUC for capecitabine than the Caucasian patients (n=22). Japanese patients had also about 25% lower Cmax and 34% lower AUC for FBAL than the Caucasian patients. The clinical significance of these differences is unknown. No significant differences occurred in the exposure to other metabolites (5'-DFCR, 5'-DFUR, and 5-FU).
- Effect of Hepatic Insufficiency
- Capecitabine has been evaluated in 13 patients with mild to moderate hepatic dysfunction due to liver metastases defined by a composite score including bilirubin, AST/ALT and alkaline phosphatase following a single 1255 mg/m2 dose of Capecitabine. Both AUC0-∞ and Cmax of capecitabine increased by 60% in patients with hepatic dysfunction compared to patients with normal hepatic function (n=14). The AUC0-∞ and Cmax of 5-FU were not affected. In patients with mild to moderate hepatic dysfunction due to liver metastases, caution should be exercised when Capecitabine is administered. The effect of severe hepatic dysfunction on Capecitabine is not known.
- Effect of Renal Insufficiency
- Following oral administration of 1250 mg/m2 capecitabine twice a day to cancer patients with varying degrees of renal impairment, patients with moderate (creatinine clearance = 30 to 50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment showed 85% and 258% higher systemic exposure to FBAL on day 1 compared to normal renal function patients (creatinine clearance >80 mL/min). Systemic exposure to 5'-DFUR was 42% and 71% greater in moderately and severely renal impaired patients, respectively, than in normal patients. Systemic exposure to capecitabine was about 25% greater in both moderately and severely renal impaired patients.
- Effect of Capecitabine on the Pharmacokinetics of Warfarin
- In four patients with cancer, chronic administration of capecitabine (1250 mg/m2 bid) with a single 20 mg dose of warfarin increased the mean AUC of S-warfarin by 57% and decreased its clearance by 37%. Baseline corrected AUC of INR in these 4 patients increased by 2.8-fold, and the maximum observed mean INR value was increased by 91%.
- Effect of Antacids on the Pharmacokinetics of Capecitabine
- When Maalox® (20 mL), an aluminum hydroxide- and magnesium hydroxide-containing antacid, was administered immediately after Capecitabine (1250 mg/m2, n=12 cancer patients), AUC and Cmax increased by 16% and 35%, respectively, for capecitabine and by 18% and 22%, respectively, for 5'-DFCR. No effect was observed on the other three major metabolites (5'-DFUR, 5-FU, FBAL) of Capecitabine.
- Effect of Capecitabine on the Pharmacokinetics of Docetaxel and Vice Versa
- A Phase 1 study evaluated the effect of Capecitabine on the pharmacokinetics of docetaxel (Taxotere®) and the effect of docetaxel on the pharmacokinetics of Capecitabine was conducted in 26 patients with solid tumors. Capecitabine was found to have no effect on the pharmacokinetics of docetaxel (Cmax and AUC) and docetaxel has no effect on the pharmacokinetics of capecitabine and the 5-FU precursor 5'-DFUR.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Adequate studies investigating the carcinogenic potential of Capecitabine have not been conducted. Capecitabine was not mutagenic in vitro to bacteria (Ames test) or mammalian cells (Chinese hamster V79/HPRT gene mutation assay). Capecitabine was clastogenic in vitro to human peripheral blood lymphocytes but not clastogenic in vivo to mouse bone marrow (micronucleus test). Fluorouracil causes mutations in bacteria and yeast. Fluorouracil also causes chromosomal abnormalities in the mouse micronucleus test in vivo.
- Impairment of Fertility
- In studies of fertility and general reproductive performance in female mice, oral capecitabine doses of 760 mg/kg/day (about 2300 mg/m2/day) disturbed estrus and consequently caused a decrease in fertility. In mice that became pregnant, no fetuses survived this dose. The disturbance in estrus was reversible. In males, this dose caused degenerative changes in the testes, including decreases in the number of spermatocytes and spermatids. In separate pharmacokinetic studies, this dose in mice produced 5'-DFUR AUC values about 0.7 times the corresponding values in patients administered the recommended daily dose
Clinical Studies
- Adjuvant Colon Cancer
- A multicenter randomized, controlled phase 3 clinical trial in patients with Dukes' C colon cancer (X-ACT) provided data concerning the use of Capecitabine for the adjuvant treatment of patients with colon cancer. The primary objective of the study was to compare disease-free survival (DFS) in patients receiving Capecitabine to those receiving IV 5-FU/LV alone. In this trial, 1987 patients were randomized either to treatment with Capecitabine 1250 mg/m2 orally twice daily for 2 weeks followed by a 1-week rest period, given as 3-week cycles for a total of 8 cycles (24 weeks) or IV bolus 5-FU 425 mg/m2 and 20 mg/m2 IV leucovorin on days 1 to 5, given as 4-week cycles for a total of 6 cycles (24 weeks). Patients in the study were required to be between 18 and 75 years of age with histologically-confirmed Dukes' stage C colon cancer with at least one positive lymph node and to have undergone (within 8 weeks prior to randomization) complete resection of the primary tumor without macroscopic or microscopic evidence of remaining tumor. Patients were also required to have no prior cytotoxic chemotherapy or immunotherapy (except steroids), and have an ECOG performance status of 0 or 1 (KPS ≥ 70%), ANC ≥ 1.5×109/L, platelets ≥ 100×109/L, serum creatinine ≤ 1.5 ULN, total bilirubin ≤ 1.5 ULN, AST/ALT ≤ 2.5 ULN and CEA within normal limits at time of randomization.
- The baseline demographics for Capecitabine and 5-FU/LV patients are shown in Table 10. The baseline characteristics were well-balanced between arms.
- All patients with normal renal function or mild renal impairment began treatment at the full starting dose of 1250 mg/m2 orally twice daily. The starting dose was reduced in patients with moderate renal impairment (calculated creatinine clearance 30 to 50 mL/min) at baseline. Subsequently, for all patients, doses were adjusted when needed according to toxicity. Dose management for Capecitabine included dose reductions, cycle delays and treatment interruptions (see Table 11).
- The median follow-up at the time of the analysis was 83 months (6.9 years). The hazard ratio for DFS for Capecitabine compared to 5-FU/LV was 0.88 (95% C.I. 0.77 – 1.01) (see Table 12 and Figure 1). Because the upper 2-sided 95% confidence limit of hazard ratio was less than 1.20, Capecitabine was non-inferior to 5-FU/LV. The choice of the non-inferiority margin of 1.20 corresponds to the retention of approximately 75% of the 5-FU/LV effect on DFS. The hazard ratio for Capecitabine compared to 5-FU/LV with respect to overall survival was 0.86 (95% C.I. 0.74 – 1.01). The 5-year overall survival rates were 71.4% for Capecitabine and 68.4% for 5-FU/LV (see Figure 2).
- Metastatic Colorectal Cancer
- General
- The recommended dose of Capecitabine was determined in an open-label, randomized clinical study, exploring the efficacy and safety of continuous therapy with capecitabine (1331 mg/m2/day in two divided doses, n=39), intermittent therapy with capecitabine (2510 mg/m2/day in two divided doses, n=34), and intermittent therapy with capecitabine in combination with oral leucovorin (LV) (capecitabine 1657 mg/m2/day in two divided doses, n=35; leucovorin 60 mg/day) in patients with advanced and/or metastatic colorectal carcinoma in the first-line metastatic setting. There was no apparent advantage in response rate to adding leucovorin to Capecitabine; however, toxicity was increased. Capecitabine, 1250 mg/m2 twice daily for 14 days followed by a 1-week rest, was selected for further clinical development based on the overall safety and efficacy profile of the three schedules studied.
- Monotherapy
- Data from two open-label, multicenter, randomized, controlled clinical trials involving 1207 patients support the use of Capecitabine in the first-line treatment of patients with metastatic colorectal carcinoma. The two clinical studies were identical in design and were conducted in 120 centers in different countries. Study 1 was conducted in the US, Canada, Mexico, and Brazil; Study 2 was conducted in Europe, Israel, Australia, New Zealand, and Taiwan. Altogether, in both trials, 603 patients were randomized to treatment with Capecitabine at a dose of 1250 mg/m2 twice daily for 2 weeks followed by a 1-week rest period and given as 3-week cycles; 604 patients were randomized to treatment with 5-FU and leucovorin (20 mg/m2 leucovorin IV followed by 425 mg/m2 IV bolus 5-FU, on days 1 to 5, every 28 days).
- In both trials, overall survival, time to progression and response rate (complete plus partial responses) were assessed. Responses were defined by the World Health Organization criteria and submitted to a blinded independent review committee (IRC). Differences in assessments between the investigator and IRC were reconciled by the sponsor, blinded to treatment arm, according to a specified algorithm. Survival was assessed based on a non-inferiority analysis.
- The baseline demographics for Capecitabine and 5-FU/LV patients are shown in Table 13.
- Capecitabine was superior to 5-FU/LV for objective response rate in Study 1 and Study 2. The similarity of Capecitabine and 5-FU/LV in these studies was assessed by examining the potential difference between the two treatments. In order to assure that Capecitabine has a clinically meaningful survival effect, statistical analyses were performed to determine the percent of the survival effect of 5-FU/LV that was retained by Capecitabine. The estimate of the survival effect of 5-FU/LV was derived from a meta-analysis of ten randomized studies from the published literature comparing 5-FU to regimens of 5-FU/LV that were similar to the control arms used in these Studies 1 and 2. The method for comparing the treatments was to examine the worst case (95% confidence upper bound) for the difference between 5-FU/LV and Capecitabine, and to show that loss of more than 50% of the 5-FU/LV survival effect was ruled out. It was demonstrated that the percent of the survival effect of 5-FU/LV maintained was at least 61% for Study 2 and 10% for Study 1. The pooled result is consistent with a retention of at least 50% of the effect of 5-FU/LV. It should be noted that these values for preserved effect are based on the upper bound of the 5-FU/LV vs Capecitabine difference. These results do not exclude the possibility of true equivalence of Capecitabine to 5-FU/LV (see Table 14, Table 15, and Figure 3).
- Breast Cancer
- Capecitabine has been evaluated in clinical trials in combination with docetaxel (Taxotere®) and as monotherapy.
- In Combination With Docetaxel
- The dose of Capecitabine used in the phase 3 clinical trial in combination with docetaxel was based on the results of a phase 1 study, where a range of doses of docetaxel administered in 3-week cycles in combination with an intermittent regimen of Capecitabine (14 days of treatment, followed by a 7-day rest period) were evaluated. The combination dose regimen was selected based on the tolerability profile of the 75 mg/m2 administered in 3-week cycles of docetaxel in combination with 1250 mg/m2 twice daily for 14 days of Capecitabine administered in 3-week cycles. The approved dose of 100 mg/m2 of docetaxel administered in 3-week cycles was the control arm of the phase 3 study.
- Capecitabine in combination with docetaxel was assessed in an open-label, multicenter, randomized trial in 75 centers in Europe, North America, South America, Asia, and Australia. A total of 511 patients with metastatic breast cancer resistant to, or recurring during or after an anthracycline-containing therapy, or relapsing during or recurring within 2 years of completing an anthracycline-containing adjuvant therapy were enrolled. Two hundred and fifty-five (255) patients were randomized to receive Capecitabine 1250 mg/m2 twice daily for 14 days followed by 1 week without treatment and docetaxel 75 mg/m2 as a 1-hour intravenous infusion administered in 3-week cycles. In the monotherapy arm, 256 patients received docetaxel 100 mg/m2 as a 1-hour intravenous infusion administered in 3-week cycles. Patient demographics are provided in Table 16.
- Capecitabine in combination with docetaxel resulted in statistically significant improvement in time to disease progression, overall survival and objective response rate compared to monotherapy with docetaxel as shown in Table 17, Figure 4, and Figure 5.
- Monotherapy
- The antitumor activity of Capecitabine as a monotherapy was evaluated in an open-label single-arm trial conducted in 24 centers in the US and Canada. A total of 162 patients with stage IV breast cancer were enrolled. The primary endpoint was tumor response rate in patients with measurable disease, with response defined as a ≥50% decrease in sum of the products of the perpendicular diameters of bidimensionally measurable disease for at least 1 month. Capecitabine was administered at a dose of 1255 mg/m2 twice daily for 2 weeks followed by a 1-week rest period and given as 3-week cycles. The baseline demographics and clinical characteristics for all patients (n=162) and those with measurable disease (n=135) are shown in Table 18. Resistance was defined as progressive disease while on treatment, with or without an initial response, or relapse within 6 months of completing treatment with an anthracycline-containing adjuvant chemotherapy regimen.
- Antitumor responses for patients with disease resistant to both paclitaxel and an anthracycline are shown in Table 19.
- For the subgroup of 43 patients who were doubly resistant, the median time to progression was 102 days and the median survival was 255 days. The objective response rate in this population was supported by a response rate of 18.5% (1 CR, 24 PRs) in the overall population of 135 patients with measurable disease, who were less resistant to chemotherapy (see Table 18). The median time to progression was 90 days and the median survival was 306 days.
How Supplied
- 150 mg
- Color: Light peach
- Engraving: Capecitabine on one side and 150 on the other 150 mg tablets are packaged in bottles of 60 (NDC 0004-1100-20).
- 500 mg
- Color: Peach
- Engraving: Capecitabine on one side and 500 on the other 500 mg tablets are packaged in bottles of 120 (NDC 0004-1101-50).
- Storage and Handling
- Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). KEEP TIGHTLY CLOSED.
- Care should be exercised in the handling of Capecitabine. Capecitabine tablets should not be cut or crushed. The use of gloves and safety glasses is recommended to avoid exposure in case of breakage of tablets. If powder from broken Capecitabine tablets contacts the skin, wash the skin immediately and thoroughly with soap and water. If Capecitabine contacts the mucous membranes, flush thoroughly with water.
- Procedures for the proper handling and disposal of anticancer drugs should be considered. Several guidelines on the subject have been published.1-4
Storage
There is limited information regarding Capecitabine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Capecitabine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Capecitabine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
- Patients and patients' caregivers should be informed of the expected adverse effects of Capecitabine, particularly nausea, vomiting, diarrhea, and hand-and-foot syndrome, and should be made aware that patient-specific dose adaptations during therapy are expected and necessary. As described below, patients taking Capecitabine should be informed of the need to interrupt treatment immediately if moderate or severe toxicity occurs. Patients should be encouraged to recognize the common grade 2 toxicities associated with Capecitabine treatment.
- Diarrhea
- Patients experiencing grade 2 diarrhea (an increase of 4 to 6 stools/day or nocturnal stools) or greater should be instructed to stop taking Capecitabine immediately. Standard antidiarrheal treatments (eg, loperamide) are recommended.
- Nausea
- Patients experiencing grade 2 nausea (food intake significantly decreased but able to eat intermittently) or greater should be instructed to stop taking Capecitabine immediately. Initiation of symptomatic treatment is recommended.
- Vomiting
- Patients experiencing grade 2 vomiting (2 to 5 episodes in a 24-hour period) or greater should be instructed to stop taking Capecitabine immediately. Initiation of symptomatic treatment is recommended.
- Hand-and-Foot Syndrome
- Patients experiencing grade 2 hand-and-foot syndrome (painful erythema and swelling of the hands and/or feet and/or discomfort affecting the patients' activities of daily living) or greater should be instructed to stop taking Capecitabine immediately.
- Stomatitis
- Patients experiencing grade 2 stomatitis (painful erythema, edema or ulcers of the mouth or tongue, but able to eat) or greater should be instructed to stop taking Capecitabine immediately. Initiation of symptomatic treatment is recommended.
- Fever and Neutropenia
- Patients who develop a fever of 100.5°F or greater or other evidence of potential infection should be instructed to call their physician.
- Patient Package Insert
- Read this leaflet before you start taking Capecitabine® [zeh-LOE-duh] and each time you refill your prescription in case the information has changed. This leaflet contains important information about Capecitabine. However, this information does not take the place of talking with your doctor. This information cannot cover all possible risks and benefits of Capecitabine. Your doctor should always be your first choice for discussing your medical condition and this medicine.
- What is Capecitabine?
- Capecitabine is a medicine you take by mouth (orally). Capecitabine is changed in the body to 5-fluorouracil (5-FU). In some patients with colon, rectum or breast cancer, 5-FU stops cancer cells from growing and decreases the size of the tumor.
- Capecitabine is used to treat:
- cancer of the colon after surgery
- cancer of the colon or rectum (colorectal cancer) that has spread to other parts of the body (metastatic colorectal cancer). You should know that in studies, other medicines showed improved survival when they were taken together with 5-FU and leucovorin. In studies, Capecitabine was no worse than 5-FU and leucovorin taken together but did not improve survival compared to these two medicines.
- breast cancer that has spread to other parts of the body (metastatic breast cancer) together with another medicine called docetaxel (TAXOTERE ®)
- breast cancer that has spread to other parts of the body and has not improved after treatment with other medicines such as paclitaxel (TAXOL ®) and anthracycline-containing medicine such as Adriamycin™ and doxorubicin
- What is the most important information about Capecitabine?
- Capecitabine may increase the effect of other medicines used to thin your blood such as warfarin (COUMADIN®). It is very important that your doctor knows if you are taking a blood thinner such as warfarin because Capecitabine may increase the effect of this medicine and could lead to serious side effects. If you are taking blood thinners and Capecitabine, your doctor needs to check more often how fast your blood clots and change the dose of the blood thinner, if needed.
- Who should not take Capecitabine?
- DO NOT TAKE Capecitabine IF YOU
- are nursing a baby. Tell your doctor if you are nursing. Capecitabine may pass to the baby in your milk and harm the baby.
- are allergic to 5-fluorouracil
- are allergic to capecitabine or to any of the ingredients in Capecitabine
- have been told that you lack the enzyme DPD (dihydropyrimidine dehydrogenase)
- DO NOT TAKE Capecitabine IF YOU
- TELL YOUR DOCTOR IF YOU
- take a blood thinner such as warfarin (COUMADIN). This is very important because Capecitabine may increase the effect of the blood thinner. If you are taking blood thinners and Capecitabine, your doctor needs to check more often how fast your blood clots and change the dose of the blood thinner, if needed.
- take phenytoin (DILANTIN®). Your doctor needs to test the levels of phenytoin in your blood more often or change your dose of phenytoin.
- are pregnant or think you may be pregnant. Capecitabine may harm your unborn child.
- have kidney problems. Your doctor may prescribe a different medicine or lower the Capecitabine dose.
- have liver problems. You may need to be checked for liver problems while you take Capecitabine.
- have heart problems because you could have more side effects related to your heart.
- take the vitamin folic acid. It may affect how Capecitabine works.
- How should I take Capecitabine?
- Take Capecitabine exactly as your doctor tells you to. Your doctor will prescribe a dose and treatment plan that is right for you. Your doctor may want you to take both 150 mg and 500 mg tablets together for each dose. If so, you must be able to identify the tablets. Taking the wrong tablets could cause an overdose (too much medicine) or underdose (too little medicine). The 150 mg tablets are light peach in color with 150 on one side. The 500 mg tablets are peach in color with 500 on one side. Your doctor may change the amount of medicine you take during your treatment. Your doctor may prescribe Capecitabine Tablets with docetaxel (TAXOTERE) injection.
- Capecitabine is taken in 2 daily doses, a morning dose and an evening dose
- Take Capecitabine tablets within 30 minutes after the end of a meal (breakfast and dinner)
- Swallow Capecitabine tablets whole with water
- If you miss a dose of Capecitabine, do not take the missed dose at all and do not double the next dose. Instead, continue your regular dosing schedule and check with your doctor.
- Capecitabine is usually taken for 14 days followed by a 7-day rest period (no drug), for a 21-day cycle. Your doctor will tell you how many cycles of treatment you will need.
- If you take too much Capecitabine, contact your doctor or local poison control center or emergency room right away.
- Take Capecitabine exactly as your doctor tells you to. Your doctor will prescribe a dose and treatment plan that is right for you. Your doctor may want you to take both 150 mg and 500 mg tablets together for each dose. If so, you must be able to identify the tablets. Taking the wrong tablets could cause an overdose (too much medicine) or underdose (too little medicine). The 150 mg tablets are light peach in color with 150 on one side. The 500 mg tablets are peach in color with 500 on one side. Your doctor may change the amount of medicine you take during your treatment. Your doctor may prescribe Capecitabine Tablets with docetaxel (TAXOTERE) injection.
- What should I avoid while taking Capecitabine?
- Women should not become pregnant while taking Capecitabine. Capecitabine may harm your unborn child. Use effective birth control while taking Capecitabine. Tell your doctor if you become pregnant.
- Do not breast-feed. Capecitabine may pass through your milk and harm your baby.
- Men should use birth control while taking Capecitabine
- What are the most common side effects of Capecitabine?
- The most common side effects of Capecitabine are:
- diarrhea, nausea, vomiting, sores in the mouth and throat (stomatitis), stomach area pain (abdominal pain), upset stomach, constipation, loss of appetite, and too much water loss from the body (dehydration). These side effects are more common in patients age 80 and older.
- hand-and-foot syndrome (palms of the hands or soles of the feet tingle, become numb, painful, swollen or red), rash, dry, itchy or discolored skin, nail problems, and hair loss
- tiredness, weakness, dizziness, headache, fever, pain (including chest, back, joint, and muscle pain), trouble sleeping, and taste problems
- The most common side effects of Capecitabine are:
- These side effects may differ when taking Capecitabine with docetaxel (TAXOTERE). Please consult your doctor for possible side effects that may be caused by taking Capecitabine with docetaxel (TAXOTERE).
- If you are concerned about these or any other side effects while taking Capecitabine, talk to your doctor.
- Stop taking Capecitabine immediately and contact your doctor right away if you have the side effects listed below, or other side effects that concern you. Your doctor can then adjust Capecitabine to a dose that is right for you or stop your Capecitabine treatment for a while. This should help to reduce the side effects and stop them from getting worse.
- Diarrhea: if you have an additional 4 bowel movements each day beyond what is normal or any diarrhea at night
- Vomiting: if you vomit more than once in a 24-hour time period
- Nausea: if you lose your appetite, and the amount of food you eat each day is much less than usual
- Stomatitis: if you have pain, redness, swelling or sores in your mouth
- Hand-and-Foot Syndrome: if you have pain, swelling or redness of your hands or feet that prevents normal activity
- Fever or Infection: if you have a temperature of 100.5°F or greater, or other signs of infection
- Your doctor may tell you to lower the dose or to stop Capecitabine treatment for a while. If caught early, most of these side effects usually improve after you stop taking Capecitabine. If they do not improve within 2 to 3 days, call your doctor again. After your side effects have improved, your doctor will tell you whether to start taking Capecitabine again and what dose to take. Adjusting the dose of Capecitabine to be right for each patient is an important part of treatment.
- How should I store and use Capecitabine?
- Never share Capecitabine with anyone
- Store Capecitabine at normal room temperature (about 65° to 85°F)
- Keep Capecitabine and all other medicines out of the reach of children
- If you take too much Capecitabine by mistake, contact your doctor or local poison control center or emergency room right away
- General advice about prescription medicines:
- Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use Capecitabine for a condition for which it was not prescribed. Do not give Capecitabine to other people, even if they have the same symptoms you have. It may harm them.
- This leaflet summarizes the most important information about Capecitabine. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Capecitabine that is written for health professionals.
Precautions with Alcohol
- Alcohol-Capecitabine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- XELODA®[9]
Look-Alike Drug Names
- Capecitabine® — Xenical®[10]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R; et al. (2008). "Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study". J Clin Oncol. 26 (12): 2013–9. doi:10.1200/JCO.2007.14.9930. PMID 18421054.
- ↑ Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L; et al. (2005). "Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer". J Clin Oncol. 23 (4): 792–9. doi:10.1200/JCO.2005.05.098. PMID 15681523.
- ↑ Schmoll HJ, Cartwright T, Tabernero J, Nowacki MP, Figer A, Maroun J; et al. (2007). "Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients". J Clin Oncol. 25 (1): 102–9. doi:10.1200/JCO.2006.08.1075. PMID 17194911.
- ↑ Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R; et al. (2008). "Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer". J Clin Oncol. 26 (12): 2006–12. doi:10.1200/JCO.2007.14.9898. PMID 18421053.
- ↑ Jatoi A, Murphy BR, Foster NR, Nikcevich DA, Alberts SR, Knost JA; et al. (2006). "Oxaliplatin and capecitabine in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction and gastric cardia: a phase II study from the North Central Cancer Treatment Group". Ann Oncol. 17 (1): 29–34. doi:10.1093/annonc/mdj063. PMID 16303863.
- ↑ Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F; et al. (2008). "Capecitabine and oxaliplatin for advanced esophagogastric cancer". N Engl J Med. 358 (1): 36–46. doi:10.1056/NEJMoa073149. PMID 18172173.
- ↑ Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W; et al. (2009). "Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer". J Clin Oncol. 27 (33): 5513–8. doi:10.1200/JCO.2009.24.2446. PMID 19858379.
- ↑ Rischin D, Phillips KA, Friedlander M, Harnett P, Quinn M, Richardson G; et al. (2004). "A phase II trial of capecitabine in heavily pre-treated platinum-resistant ovarian cancer". Gynecol Oncol. 93 (2): 417–21. doi:10.1016/j.ygyno.2004.01.037. PMID 15099955.
- ↑ "Capecitabine (capecitabine) tablet, film coated [Genentech, Inc.]".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Capecitabine |Pill Name=Xeloda_NDC_00041100.jpg |Drug Name=Xeloda |Pill Ingred=capecitabine[capecitabine]|+sep=; |Pill Imprint=XELODA;150 |Pill Dosage=150 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Genentech, Inc. |NDC=00041100
}}
{{#subobject:
|Page Name=Capecitabine |Pill Name=Xeloda_NDC_00041101.jpg |Drug Name=Xeloda |Pill Ingred=capecitabine[capecitabine]|+sep=; |Pill Imprint=XELODA;500 |Pill Dosage=500 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Genentech, Inc. |NDC=00041101
}}
{{#subobject:
|Label Page=Capecitabine |Label Name=Capecitabine20.png
}}
{{#subobject:
|Label Page=Capecitabine |Label Name=Capecitabine21.png
}}
{{#subobject:
|Label Page=Capecitabine |Label Name=Capecitabine32.png
}}
{{#subobject:
|Label Page=Capecitabine |Label Name=Capecitabine33.png
}}
}}