CMR image acquisition protocols
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Adapted from SCMR Image Acquisition Protocols v 1.0, assembled by the Society of Cardiovascular Magnetic Resonance, March 2007.
General Techniques
Equipment
Independent of the magnet and cardiac coil, the following are recommended to perform a CMR scan:
- Monitoring equipment (blood pressure, cardiac rhythm)
- Preparation and practice for rapid removal of the patient from the magnet
- Defribillator
- Drugs for emergency treatment:
- Aminophylline
- Emergency cart with full set of emergency drugs
- Beta-blockers
- Nitroglycerin
- For dobutamine scans, online assessment of wall motion during image reconstruction performed immediately after image acquisition
- Stress Agents
- Dobutamine-HCl: [] preferably 5 mg/ml, i.v. administration
- Atropine: [] 0.25 mg fractions (max dose: 2 mg)
- Adenosine: [] 30 ml vial (dose as per package insert: 140 μg/kg/min)
Contraindications
- Dobutamine
- Severe arterial hypertension (≥220/120 mmHg)
- Unstable angina pectoris
- Significant aortic stenosis (aortic valve gradient >50 mmHg or aortic valve area <1 cm²)
- Complex cardiac arrhythmias
- Obstructive hypertrophic cardiomyopathy
- Myocarditis, endocarditis, pericarditis
- Adenosine
- Known or suspeced bronchoconstrictive or bronchospastic disease
- 2nd or 3rd degree AV block
- Sinus bradycardia (HR <45 bpm)
- Systemic hypotension (< 90 mmHg)
Patient Preparation
- Informed consent must be obtained for CMR stress tests
- Patients should refrain from the following medications for at least 24 hours prior to examination, in order to prevent stress agent interactions and complications:
- Dobutamine: B-blockers and nitrates
- Adenosine: caffeine, theophylline
- There is no need for fasting
- To minimize respiratory motion, patients should dispose of any chewing gum, hard candies, cough drops before entering the scanner
Potential Adverse Effects
- Dobutamine at high doses may cause chest pain, palpitations. More severe complications occur in 0.25% of patients including:
- Infarction (0.07%)
- Ventricular fibrillation (0.07%)
- Sustained ventricular tachycardia (0.1%)
- Adenosine may cause flushing, chest pain, palpitations. More severe complications include:
- Heart block
- Hypotension
- Sinus tachycardia
- Bronchospasm
Left Ventricle Structure and Function Module
The following sequences are required to accurately assess left ventricular (LV) structure and function:
- Scout images in the following planes:
- Axial
- Coronal
- Sagittal
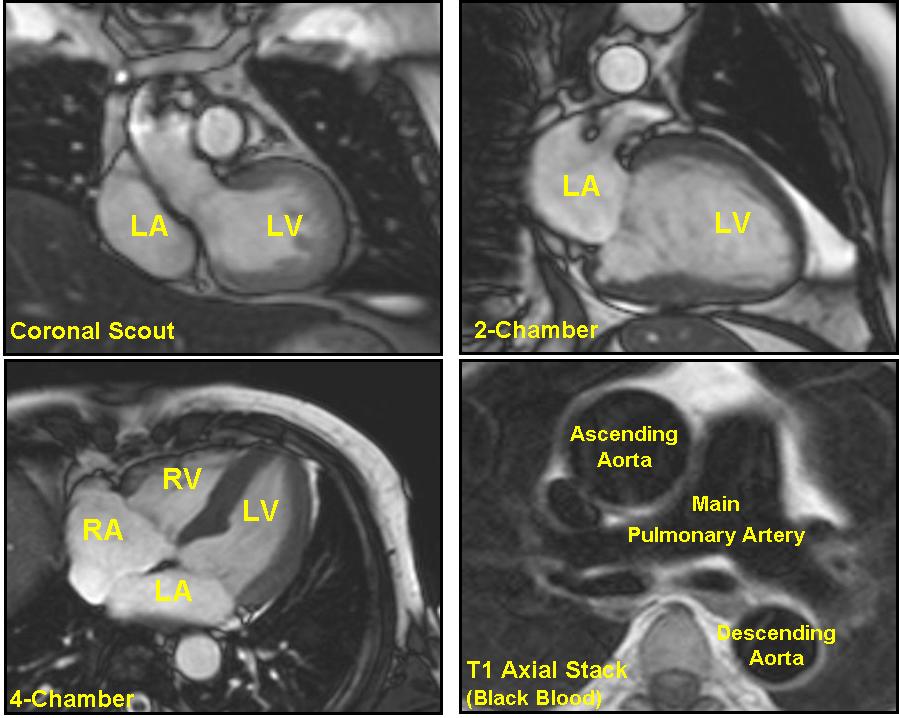
- Axial set (8-10 mm) of steady state free precession (SSFP) or half Fourier single shot turbo spin echo images through the chest
- Scout to line up short axis images (can either be a single shot or cine acquisition)
- 2-chamber long axis (also called the vertical long axis) prescribed off an axial view showing the apex and mitral valve, bisecting the mitral valve and apex
- Horizontal long axis prescribed off of the 2-chamber long axis, again bisecting the apex and center of the mitral valve
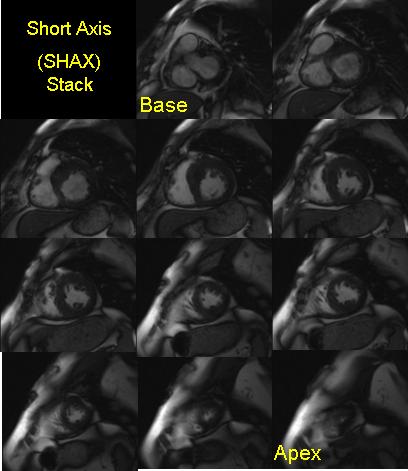
- Steady state free precessions short axis cine images, from the mitral valve plane through the apex prescribed from the previously acquired horizontal long axis image
- Slice thicknesses: 6-8 mm, with 2 mm interslice gap; or 8-10 mm with no interslice gap
- Temporal resolution <45 msec between phases
- Parallel imaging used as available, speed up factor 2x
- Steady state free precession long axis images
- 4-chamber long axis, prescribed off a basal short axis image, bisecting the interventricular septum
- 2-chamber long axis, prescribed off of a basal short axis image, bisecting the anterior and inferior walls
- 3-chamber long axis, prescribed off of the most basal short axis including the plane of the LVOT, bisecting the LVOT and posterolateral wall
CMR Analysis of the Left Ventricle:
- All short axis images are evaluated with computer aided analysis packages for planimetry of endocardial and epicardial borders at end-diastole and end-systole
- The inclusion or exclusion of papillary muscles and trabeculations in the LVass should be the same as that used in normal reference ranges used for comparison
- Special care must be used in evaluating the 1 or 2 most basal slices. Due to systolic movement of the base towards the apex, the end-systolic phase will include only left atrium. However, this slice at end-diastole will include some of the LV mass and volume
Gadolinium Dosing Module
Indication Contrast Dose (mmol/Kg body weight unless otherwise stated) Injection Rate NaCl Injection Rate Perfusion 0.05-0.1 3-5 ml/sec 30 ml 4 ml/sec Late Gadolinium Enhancement 0.15-0.2 3-5 ml/sec 20 ml 4 ml/sec Angiography (Carotids, Renals, Thoracic or Abdominal Aorta) 0.1-0.2 2-3 ml/sec 20 ml 2-3 ml/sec Time-Resolved Angiography 10 ml 3-5 ml/sec 30 ml 4 ml/sec Peripheral Angiography 0.2 mM/kg First 10 ml @ 1.5 ml/sec Rest @ 0.4-0.8 ml/sec 20 ml 0.4-0.8 ml/sec
Some notes to consider:
- Volumes and injection rates depend on scan duration: given values are recommendations for standard scan times
- Injection rates are different for 1 molar contrast agents. General Rule: divide the given injection rates by a factor of 2
- Contrast agents with higher relaxivity (e.g. gadobenate dimeglumine [Multihance] require smaller doses)
- Throughout the protocols, the term "gadolinium" refers to gadolinium chelates
First Pass Perfusion Module
- Scout imaging as per LV structure and function module
- Saturation-recovery imaging with GRE-EPI hypbrid, GRE or SSFP readout
- Short-axis view imaging (at least 3 slices per heartbeat):
- For ischemia evaluation, must obtain data every heart beat
- Slice thickness of 8-10 mm
- Parallel imaging, 2-fold acceleration if available
- In-plane resolution, ~2-3 mm
- Readout temporal resolution ~100-125 ms or short as available
- Contrast is given (0.05-0.1 mM/kg, 3-5 ml/sec) followed by at least 30 ml saline flush (3-7 ml/sec)
- Breathold starts during early phases of contrast infusion before contrast reaches the myocardium
- Image for 40-50 heart beats by which time contrast has passed through the LV myocardium
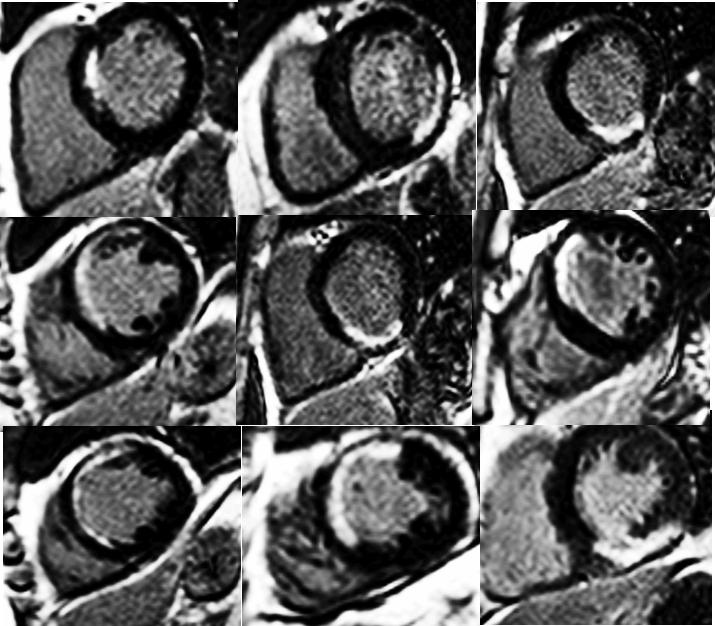
Late Gadolinium Enhancement Module
- Need at least 10 minute wait after contrast administration (0.15-0.2 mmol/kg)
- Note: The delay may be shorter than 10 minutes if lower doses are used as blood pool signal falls below that of late enhanced myocardium
- 2D segmented inversion recovery GRE imaging during diastolic stand-still
- Same views as for cine imaging (short- and long-axis views)
- Slice thickness, same as for cine imaging
- In-plane resolution, ~1.4-1.8 nm
- Acquisition duration per R-R interval below 200 msec but should be less in the setting of tachycardia
- Inversion time set to null normal myocardium. Alternative is to use fixed T1 with a phase-sensitive sequence
- Readout is usually every other heart beat but can be modified to every heart beat in the setting of bradycardia, and every third heart beat in the setting of tachycardia or arrhythmia
- Optional:
- Single-shot imaging (SSFP readout) performed as backup for patients with irregular heart beat, difficulty breath holding
- 3D sequences with parallel imaging in appropriate patients
- Analysis:
- Interpret areas of enhancement using AHA 17-segment model
- Estimate area (transmural extent) of enhancement within each segment (0%, 1-25%, 26-50%, 76-100%)
- Some analysis software enables for quantification of enhancement by applying various thresholding methodologies, depending upon the cardiac condition
Disease Specific Protocols
Acute Myocardial Infarction
- LV structure and function module
- Optional: T2 weighted black blood imaging (at least areas with all motion abnormalities)
- See Nonischemic Cardiomyopathies for sequence
- First pass perfusion
- To look for microvascular obstruction, consider repeat first pass perfusion sequence or early gadolinium enhancement, i.e. within the first 2-3 minutes after contrast infusion
- Late gadolinium enhancement module
Chronic Ischemic Heart Disease and Viability
- LV structure and function module
- Optional: Adenosine stress-rest perfusion or high dose dobutamine functional imaging to comment on the presence or absence of ischemia
- Late gadolinium enhancement module
- Optional: Low dose dobutamine: 5-10 minute infusion of 10 μg/kg/min of dobutamine to assess contractile reserve as improvement in wall thickening
- Analysis:
- Helpful to view cines and late gadolinium enhancement images side-by-side
- Interpret using both cine and late gadolinium enhancement data (e.g. "region is dysfunctional, but viable")
Dobutamine Stress MR
- LV structure and function module
- Dobutamine stimulation in increments of 10 μg/kg bodyweight/minute every 3 minutes until target heart rate is reached. Add atropine, if target heart rate is not reached at 40 μg dobutamine. Repeat 3 short axis and 3 long axis cine views during each increment. View cine loops online. Adapt heart rate as needed. Stop test for new wall motion abnormality, serious side effect, or achievement of peak heart rate.
- Analysis:
- View cines in "quadscreen" format, reviewing rest, intermediate stress levels and peak stress at the same time in synchronized fashion
- Describe wall motion as normokinetic, mild hypokinetic, severe hypokinetic, akinetic and dyskinetic for all 17 segments
- Look for ischemia and viability
Adenosine Stress Perfusion CMR
- LV structure and function module (can be performed between stress and rest perfusion)
- Two intravenous lines should be available, one for contrast and one for adenosine, one in each arm. Preferential site of contrast infusion is antecubital. Blood pressure cuff should be used with care taken not to interfere with infusion
- Adenosine stress perfusion imaging (at least 3-4 minute infusion of 140 μg/kg/min). Option:initial adenosine infusion may be performed with the patient outside the bore of the magnet
- First pass perfusion module
- During last minute of adenosine, contrast is given
- After imaging for 40-50 heart beats by which time contrast has passed through the LV myocardium, adenosine is stopped.
- Rest perfusion
- Need ~10 min wait for contrast to washout from stress perfusion imaging. During this period stress images can be reviewed, cine imaging can be completed (e.g. long-axis views), valvular evaluation can be performed, etc.
- Perfusion imaging repeated without adenosine using same dose of gadolinium contrast
- If stress images are normal, rest perfusion can be skipped and additional gadolinium may be given as needed for late gadolinium enhanced imaging (for a total of 0.15-0.2 mM/kg)
- Late gadolinium enhancement module
- Need to wait at least 5 minutes after rest perfusion
- Analysis
- Interpret visually using 17-segment AHA model (16-segment model can be used, leaving out apex)
- Helpful to view cines, stress and rest perfusion, and DE images all side-by-side
Peripheral MRA
- Peripheral vascular coil or combination of coils, as available. Venous compression cuffs (placed on the thighs, and inflated to 60 mmHg) are helpful, if available
- Axial, low resolution, vessel scoutnig with time-of-flight MRA or SSFP
- Contrast timing
- Option 1: Axial test bolus at level of distal abdominal aorta. 2 cc injection of Gadolinium (Gd)-chelate, followed by 20 cc saline. Determine time to peak contrast enhancement following injection
- Option 2: Automatic triggering technique to time start of scan
- Stepping-table, contrast-enhanced MRA performed in the coronal projection from the mid abdominal aorta to the feet
- Gd-chelate injected in 2 phases to minimize venous contamination followed by saline bolus
- Slice thickness 1-1.5 mm; acquired spatial resolution in-plane 0.8-1.5 mm
- Slices: Typically 60-80, as needed to accomodate vessels of interest.
- Volumes are obtained of abdomen/pelvis and thighs may be coarser spatial resolution (larger vessels), while those of the legs preferably are sub-millimeter spatial resolution. The former acquisitions typically require 15-20 seconds, while the leg acquisition may take 60-90 seconds for increased spatial resolution. Elliptical centric k-space acquisition is advantageous for the legs. If available, time-resolved acquisitions are preferred for the legs.
- Parallel acquisition recommended (multichannel surface coil needed)
- 2 volumetric acquisitions: One pre-contrast (for subtraction) and one during contrast administration
- Analysis
- MIP and MPR reconstructions are performed in the orthogonal views for each station. Contrast-enhanced MRA at each station is evaluated qualitatively by scrolling through the coronal slices. The presence, number, and degree of stenoses are evaluated qualitatively.
Alternative: Dual Injection Protocol
- Single dose of Gd: Time-resolved MRA of the calf and foot vessels
- Single dose of Gd: Abdominal and thigh vessels
Thoracic Aortic MRA
- Localizer, 3 orientations
- Half-fourier single shot TSE or SSFP (one breathold, entire thorax). Axial orientation
- Axial T1-weighted TSE through aorta (for intramural hematoma, dissection)
- SSFP cine imaging in parasagittal plane parallel to aorta
- Option: Use 3-point piloting
- Evaluate aortic valve as per valvular protocol
- Contrast timing
- Option 1: Axial test bolus at level of distal abdominal aorta. 2 cc injection of Gadolinium (Gd)-chelate, followed by 20 cc saline. Determine time to peak contrast enhancement following injection.
- Option 2: Automatic triggering technique to time start of scan
- Option 3: Rapid multiphase 3D acquisitions without timing sequences
- 3D contrast enhanced MRA (0.1-0.2 mmol/kg Gd-based contrast) (Optional: ECG-gated acquisition)
- Use spatial resolutiong at least 1-1.5 mm
- Parallel acquisition if available
- At least 2 acquisitions after contrast injection
- Optional: Axial T1 weighted gradient-echo post contrast for aortitis
- Analysis: MPR reconstruction, MIP and thin slab MIP
Anomalous Coronary Artery Evaluation
- LV structure and function module to look for wall motion abnormalities
- Add repeat 4-chamber long axis with high temporal resolution sequence (≤20 msec per phase) to accurately determine quiescent period of RCA
- Navigator-gated, 3D, free-breathing, MRA sequence:
- Axial slices spanning from level of proximal main pulmonary artery down to the middle of the right atrium (entire cardiac coverage if desired)
- Slice thickness 1-1.5 mm; acquired spatial resolution in-plane approximately 1.0 mm
- Slices: Typically 50-80, as needed to encompass vessels of interest.
- Parallel acquisition preferred
- Navigator placed over the right hemi-diaphragm
- Optional: Contrast to increase vessel conspicuity
- Optional:
- Breathold techniques if poor image quality or navigators unavailable or of poor quality
- T2-prepared sequence may be useful
Pulmonary Vein Evaluation (Pre- and Post-Ablation)
- LV structure and function module (if pre-ablation)
- Breathold nongated contrast-enhanced MRA performed in the coronal projection encompassing the pulmonary veins and left atrium (greater anterior coverage if breatholding permits) (Optional: Optimize oblique projections, ECG-gated acquisition)
- Gadolinium (Gd)-chelate (0.1-0.2 mM/kg) injected at 2-3 cc/sec
- Slice thickness 1-2 mm; acquired spatial resolution in-plane 1-1.5 mm
- Slices: Typically 60-80, as needed to encompass region of interest
- Parallel acquisition used as available (multichannel surface coil needed)
- 2-3 volumetric acquisitions-Each breathold typically no longer than 15-18 seconds
- Optional: Through plane phase contrast flow analysis through each pulmonary vein
- Analysis:
- Contrast-enhanced MRA evaluated qualitatively by scrolling through coronal slices.
- The number and position of pulmonary veins is accounted for noting common trunks, accessory veins, and evidence for stenosis or thrombosis
- A 3D workstation is used for MPR analysis to calculate major and minor axes, and cross-sectional area of each pulmonary vein ostium. Compare pre- and post-ablation images side by side.
Nonischemic LV Cardiomyopathies
- LV structure and function module
- Consider T2-weighted black blood imaging in the acute setting when necrosis/edema may be present (e.g. myocarditis)
- Breath-hold, segmented fast spin-echo imaging with dark blood preparation (double inversion recovery)
- Perform imaging prior to contrast administration
- Selected slices based on cine imaging findings (e.g. 2- and 4-chamber long axis and 3 representative short axis slices)
- Adjust read-out to mid-diastole
- Slice thickness 8-10 mm
- Slice thickness of dark blood prep should be greater than the base-apex motion of the mitral annulus
- Late gadolinium enhancement module
- Analysis: Examine the "pattern" of enhancement as certain nonischemic cardiomyopathies have predilection for scarring in various myocardial regions
- Optional: Adenosine stress-rest perfusion imaging or high dose dobutamine stress functional imaging (see stress protocols) to comment on the presence or absence of ischemia since a mixed cardiomyopathy may be present
- For hypertrophic cardiomyopathy, consider LV outflow tract flow tract imaging (especially in obstructive hypertrophic cardiomyopathy)
Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC)
- LV structure and function module
- Consider 5-6 mm slice thickness
- Volumetric analysis of LV and RV is helpful
- Axial or oblique axial SSFP cine images covering RV including RVOT, RV long axis view may be helpful
- Optional sequences:
- Selected axial or oblique axial black blood images (double inversion recovery TSE)
- Repeat same geometry with fat suppression
- Late gadolinium enhancement module in same orientations as above. Consider T1 nulling for RV
- Consider use of anterior surface coil only to improve resolution without "wrap around" artifacts
- CMR can add 1 major or 1 minor criterion (right ventricular enlargement, right ventricular wall motion abnormalities or aneurysms). A major finding is an easily visualized severe abnormality, a minor finding is a moderate abnormality which may be less clear. Fat and late gadolinium enhancement are not used in the guidelines.
Congenital Heart Disease
For all diagnoses:
- LV structure and function module
- Add axial SSFP cine imaging from the inferior wall of the LV through the top of the aortic arch with 8 mm thick slices with a 2 mm gap (or 10 mm and no interslice gap)
- Gradient echo cine or hybrid gradient echo/echo planar imaging may be added for improved detection of turbulent flow in particular planes
- Scout imaging in the plane of the ascending aorta and main pulmonary artery
- Through plane velocity encoded cine imaging through main pulmonary arter and aorta to measure Qp and Qs
- Time resolved or multiple rapid dynamic 3D gadolinium enhanced magnetic resonance angiography in a coronal orientation
For individual diagnoses, protocols must be individualized. Additional sequences to consider:
- For shunt lesions:
- In plan and/or through plane velocity encoded gradient echo sequence through the shunt
- For lesions involving great vessels:
- SSFP cine imaging in parasagittal plane parallel to aorta
- Assessment of valves as per valvular protocol
- SSFP cine and/or gradient echo cine images along the individual pulmonary arteries
Valvular Disease
Patients with artificial valves can safely undergo CMR at 1.5 and 3 Tesla. The force exerted by the beating heart is many fold higher than the force exerted by the magnetic field.
- LV structure and function module
- Use 4-chamber to look for valve anatomy and turbulence of the mitral and tricuspid valve
- 3-chamber for mitral and aortic valve
- 2-chamber for mitral valve
- Coronal view for aortic valve
- Additional views (RV long axis, RV-outflow tract as needed)
- Optional:
- Valve morphology assessment with SSFP cine in the plane of the valve in question. Particular care must be taken to optimize angle of imaging and to take a full stack of images across the valve.
- Gradient echo or hybrid echo/echo planar imaging-More sensitive for milder degrees of regurgitation
- Measure flow velocity and volume perpendicular to the vessel distal to valve leaflet tips
- Adapt velocity encoding to actual velocity (using lowest velocity without aliasing)
- Use lowest TE possible
- Normalize velocities to reference static tissue
- Analysis:
- Determine left and right ventricular stroke volume to measure single valve regurgitation
- Mitral regurgitation can be measured by subtracting aortic flow from the the LV stroke volume
- Multiple valve lesions can be assessed from comparison of the aortic and pulmonary diastolic regurgitant flow and the LV and RV stroke volumes
- Calculate aortic valve area by:
- (Velocity time integral LVOT/Velocity time integral aorta) x Area LVOT
- Calculate peak mitral valve gradient from peak mitral valve flow:
- MVA = 220/pressure half time
Pericardial Disease
- LV structure and function module
- T1- or T2-weighted black blood turbo spin echo images
- 2-3 representative long axis images and 3-4 representative short axis images
- If pericardial cyst is in differential, refer to masses protocol
- Optional:
- If regions of thickened pericardium noted, T1-weighted gradient echo myocardial tagged cine sequences to demonstrate presence or absence of epicardial/pericardial slippage (2-3 long axis images and 1-2 short axis images)
- Encouraged if available: Real-time imaging in the short axis during dynamic breathing maneuvers for evaluation of ventricular interdependence
- Late gadolinium enhancement module
Cardiac and Paracardiac Masses (including Thrombi)
- LV structure and function module
- T1-weighted turbo spin echo
- 1-3 slices through the mass
- T2-weighted turbo spin echo with fat suppression (Optional: Without fat supression)
- 1-3 slices through the mass
- See nonischemic cardiomyopathies for sequence details
- First pass perfusion module with slices through mass
- Repeat T1 weighted turbo spin echo
- Optional: Repeat selected steady state free precession cine images post-contrast
- Late gadolinium enhancement module