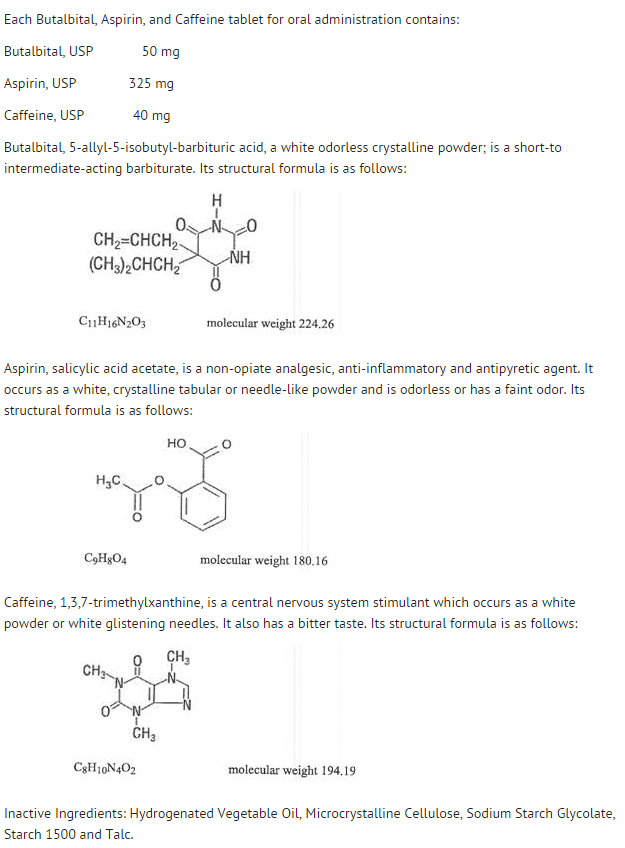
Butalbital: Difference between revisions
No edit summary |
No edit summary |
||
| Line 47: | Line 47: | ||
=====Condition1===== | =====Condition1===== | ||
* | * Butalbital, aspirin, and caffeine tablets are indicated for the relief of the symptom complex of tension (or muscle contraction) headache. | ||
Evidence supporting the efficacy and safety of butalbital, aspirin, and caffeine in the treatment of multiple recurrent headaches is unavailable. Caution in this regard is required because butalbital is habit-forming and potentially abusable. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 161: | Line 143: | ||
|contraindications= | |contraindications= | ||
* | *Hypersensitivity to aspirin, caffeine, or barbiturates. Patients with porphyria. | ||
<!--Warnings--> | <!--Warnings--> | ||
| Line 167: | Line 149: | ||
|warnings= | |warnings= | ||
* | =====Drug Dependency:===== | ||
*Prolonged use of barbiturates can produce drug dependence, characterized by psychic dependence, and less frequently, physical dependence and tolerance. The abuse liability of butalbital, aspirin, and caffeine is similar to that of other barbiturate-containing drug combinations. Caution should be exercised when prescribing medication for patients with a known propensity for taking excessive quantities of drugs, which is not uncommon in patients with chronic tension headache. | |||
=====Use in Ambulatory Patients:===== | |||
*Butalbital, aspirin, and caffeine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly. Central Nervous System depressant effects of butalbital may be additive with those of other CNS depressants. Concurrent use with other sedative-hypnotics or alcohol should be avoided. When such combined therapy is necessary, the dose of one or more agents may need to be reduced. | |||
=====Precautions===== | |||
*Salicylates should be used with extreme caution in the presence of peptic ulcer or coagulation abnormalities. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
| Line 178: | Line 165: | ||
|clinicalTrials= | |clinicalTrials= | ||
*The most frequent adverse reactions are drowsiness and dizziness. Less frequent adverse reactions are lightheadedness and gastrointestinal disturbances including nausea, vomiting, and flatulence. A single incidence of bone marrow suppression has been reported with the use of butalbital, aspirin, and caffeine. Several cases of dermatological reactions including toxic epidermal necrolysis and erythema multiforme have been reported. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 250: | Line 171: | ||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
| Line 313: | Line 182: | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* | * Adequate studies have not been performed in animals to determine whether this drug affects fertility in males or females, has teratogenic potential, or has other adverse effects on the fetus. While there are no well-controlled studies in pregnant women, over twenty years of marketing and clinical experience does not include any positive evidence of adverse effects on the fetus. Although there is no clearly defined risk, such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus. Butalbital, aspirin, and caffeine should be used in pregnant women only when clearly needed. | ||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 324: | Line 193: | ||
|useInNursing= | |useInNursing= | ||
*The effects of butalbital, aspirin, and caffeine on infants of nursing mothers are not known. Salicylates and barbiturates are excreted in the breast milk of nursing mothers. The serum levels in infants are believed to be insignificant with therapeutic doses. | |||
|useInPed= | |useInPed= | ||
*Safety and effectiveness in pediatric patients below the age of 12 have not been established. | |||
|useInGeri= | |useInGeri= | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
| Line 374: | Line 241: | ||
|overdose= | |overdose= | ||
*The toxic effects of acute overdosage of butalbital, aspirin, and caffeine are attributable mainly to its barbiturate component, and, to a lesser extent, aspirin. Because toxic effects of caffeine occur in very high dosages only, the possibility of significant caffeine toxicity from butalbital, aspirin, and caffeine overdosage is unlikely. Symptoms attributable to acute barbiturate poisoning include drowsiness, confusion, and coma; respiratory depression; hypotension; shock. Symptoms attributable to acute aspirin poisoning include hyperpnea; acid-base disturbances with development of metabolic acidosis; vomiting and abdominal pain; tinnitus; hyperthermia; hypoprothrombinemia; restlessness; delirium; convulsions. Acute caffeine poisoning may cause insomnia, restlessness, tremor, and delirium; tachycardia and extrasystoles. Treatment consists primarily of management of barbiturate intoxication and the correction of the acid-base imbalance due to salicylism. Vomiting should be induced mechanically or with emetics in the conscious patient. Gastric lavage may be used if the pharyngeal and laryngeal reflexes are present and if less than 4 hours have elapsed since ingestion. A cuffed endotracheal tube should be inserted before gastric lavage of the unconscious patient and when necessary to provide assisted respiration. Diuresis, alkalinization of the urine, and correction of electrolyte disturbances should be accomplished through administration of intravenous fluids such as 1% sodium bicarbonate in 5% dextrose in water. Meticulous attention should be given to maintaining adequate pulmonary ventilation. Correction of hypotension may require the administration of levartherenol bitartrate or phenylephrine hydrochloride by intravenous infusion . In severe cases of intoxication, peritoneal dialysis, hemodialysis, or exchange transfusion may be lifesaving. Hypoprothrombinemia should be treated with Vitamin K, intravenously. | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | ||
| Line 438: | Line 295: | ||
|howSupplied= | |howSupplied= | ||
* | *Butalbital, Aspirin, and Caffeine Tablets, USP 50 mg/325 mg/40 mg are White, Round, Unscored Compressed Tablets Imprinted “West-ward 785”. | ||
Bottles of 30 tablets | |||
Bottles of 50 tablets | |||
Bottles of 100 tablets | |||
Bottles of 500 tablets | |||
Bottles of 1000 tablets | |||
Unit Dose Boxes of 100 tablets | |||
*Store at 20-25oC (68-77oF) [See USP Controlled Room Temperature]. Protect from light and moisture. | |||
*Dispense in tight, light-resistant container as defined in the USP using a child-resistant closure. | |||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
| Line 461: | Line 328: | ||
|lookAlike= | |lookAlike= | ||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
Revision as of 18:20, 3 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Butalbital is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Butalbital, aspirin, and caffeine tablets are indicated for the relief of the symptom complex of tension (or muscle contraction) headache.
Evidence supporting the efficacy and safety of butalbital, aspirin, and caffeine in the treatment of multiple recurrent headaches is unavailable. Caution in this regard is required because butalbital is habit-forming and potentially abusable.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Butalbital in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Butalbital in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Butalbital in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Butalbital in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Butalbital in pediatric patients.
Contraindications
- Hypersensitivity to aspirin, caffeine, or barbiturates. Patients with porphyria.
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Drug Dependency:
- Prolonged use of barbiturates can produce drug dependence, characterized by psychic dependence, and less frequently, physical dependence and tolerance. The abuse liability of butalbital, aspirin, and caffeine is similar to that of other barbiturate-containing drug combinations. Caution should be exercised when prescribing medication for patients with a known propensity for taking excessive quantities of drugs, which is not uncommon in patients with chronic tension headache.
Use in Ambulatory Patients:
- Butalbital, aspirin, and caffeine may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly. Central Nervous System depressant effects of butalbital may be additive with those of other CNS depressants. Concurrent use with other sedative-hypnotics or alcohol should be avoided. When such combined therapy is necessary, the dose of one or more agents may need to be reduced.
Precautions
- Salicylates should be used with extreme caution in the presence of peptic ulcer or coagulation abnormalities.
Adverse Reactions
Clinical Trials Experience
- The most frequent adverse reactions are drowsiness and dizziness. Less frequent adverse reactions are lightheadedness and gastrointestinal disturbances including nausea, vomiting, and flatulence. A single incidence of bone marrow suppression has been reported with the use of butalbital, aspirin, and caffeine. Several cases of dermatological reactions including toxic epidermal necrolysis and erythema multiforme have been reported.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Butalbital in the drug label.
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Adequate studies have not been performed in animals to determine whether this drug affects fertility in males or females, has teratogenic potential, or has other adverse effects on the fetus. While there are no well-controlled studies in pregnant women, over twenty years of marketing and clinical experience does not include any positive evidence of adverse effects on the fetus. Although there is no clearly defined risk, such experience cannot exclude the possibility of infrequent or subtle damage to the human fetus. Butalbital, aspirin, and caffeine should be used in pregnant women only when clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Butalbital in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Butalbital during labor and delivery.
Nursing Mothers
- The effects of butalbital, aspirin, and caffeine on infants of nursing mothers are not known. Salicylates and barbiturates are excreted in the breast milk of nursing mothers. The serum levels in infants are believed to be insignificant with therapeutic doses.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 12 have not been established.
Geriatic Use
There is no FDA guidance on the use of Butalbital with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Butalbital with respect to specific gender populations.
Race
There is no FDA guidance on the use of Butalbital with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Butalbital in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Butalbital in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Butalbital in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Butalbital in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Butalbital in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Butalbital in the drug label.
Overdosage
- The toxic effects of acute overdosage of butalbital, aspirin, and caffeine are attributable mainly to its barbiturate component, and, to a lesser extent, aspirin. Because toxic effects of caffeine occur in very high dosages only, the possibility of significant caffeine toxicity from butalbital, aspirin, and caffeine overdosage is unlikely. Symptoms attributable to acute barbiturate poisoning include drowsiness, confusion, and coma; respiratory depression; hypotension; shock. Symptoms attributable to acute aspirin poisoning include hyperpnea; acid-base disturbances with development of metabolic acidosis; vomiting and abdominal pain; tinnitus; hyperthermia; hypoprothrombinemia; restlessness; delirium; convulsions. Acute caffeine poisoning may cause insomnia, restlessness, tremor, and delirium; tachycardia and extrasystoles. Treatment consists primarily of management of barbiturate intoxication and the correction of the acid-base imbalance due to salicylism. Vomiting should be induced mechanically or with emetics in the conscious patient. Gastric lavage may be used if the pharyngeal and laryngeal reflexes are present and if less than 4 hours have elapsed since ingestion. A cuffed endotracheal tube should be inserted before gastric lavage of the unconscious patient and when necessary to provide assisted respiration. Diuresis, alkalinization of the urine, and correction of electrolyte disturbances should be accomplished through administration of intravenous fluids such as 1% sodium bicarbonate in 5% dextrose in water. Meticulous attention should be given to maintaining adequate pulmonary ventilation. Correction of hypotension may require the administration of levartherenol bitartrate or phenylephrine hydrochloride by intravenous infusion . In severe cases of intoxication, peritoneal dialysis, hemodialysis, or exchange transfusion may be lifesaving. Hypoprothrombinemia should be treated with Vitamin K, intravenously.
There is limited information regarding Chronic Overdose of Butalbital in the drug label.
Pharmacology
There is limited information regarding Butalbital Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Butalbital in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Butalbital in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Butalbital in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Butalbital in the drug label.
How Supplied
- Butalbital, Aspirin, and Caffeine Tablets, USP 50 mg/325 mg/40 mg are White, Round, Unscored Compressed Tablets Imprinted “West-ward 785”.
Bottles of 30 tablets Bottles of 50 tablets Bottles of 100 tablets Bottles of 500 tablets Bottles of 1000 tablets Unit Dose Boxes of 100 tablets
- Store at 20-25oC (68-77oF) [See USP Controlled Room Temperature]. Protect from light and moisture.
- Dispense in tight, light-resistant container as defined in the USP using a child-resistant closure.
Storage
There is limited information regarding Butalbital Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Butalbital |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Butalbital |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Butalbital in the drug label.
Precautions with Alcohol
- Alcohol-Butalbital interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
There is limited information regarding Butalbital Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Butalbital |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Butalbital |Label Name=Butalbital11.png
}}
{{#subobject:
|Label Page=Butalbital |Label Name=Butalbital11.png
}}