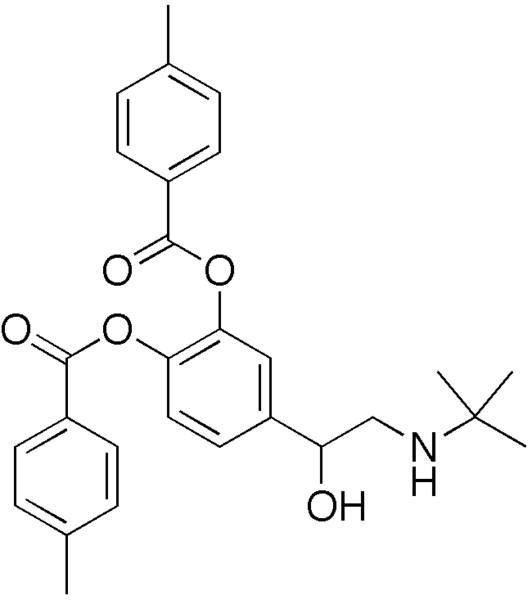
Bitolterol
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C28H31NO5 |
| Molar mass | 461.549 g/mol |
|
WikiDoc Resources for Bitolterol |
|
Articles |
|---|
|
Most recent articles on Bitolterol |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Bitolterol at Clinical Trials.gov Clinical Trials on Bitolterol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Bitolterol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Bitolterol Discussion groups on Bitolterol Patient Handouts on Bitolterol Directions to Hospitals Treating Bitolterol Risk calculators and risk factors for Bitolterol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Bitolterol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Bitolterol mesylate (Tornalate®) is a β2-adrenergic receptor agonist used for the relief of bronchospasm in conditions such as asthma and COPD.
Bitolterol has a rapid onset of action (2-5 minutes) and may last up to 6-8 hours.
Bitolterol was withdrawn from the market by Élan Pharmaceuticals in 2001.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Asthma
- Pulmonology