Bithionol: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox | |||
{{ | | Verifiedfields = changed | ||
| Watchedfields = changed | |||
| verifiedrevid = 459980346 | |||
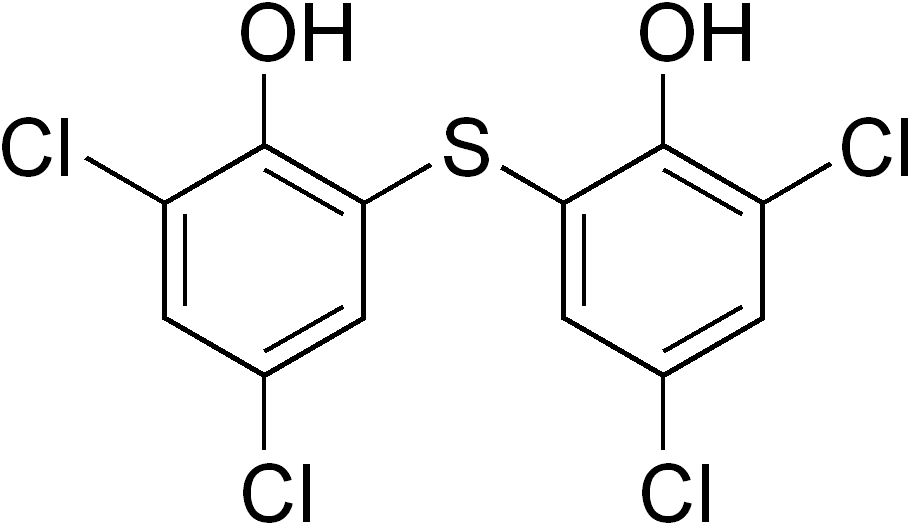
| IUPAC_name = 2,2'-sulfanediylbis(4,6-dichlorophenol) | |||
| image = Bithionol.png | |||
| image2 = Bithionol molecule ball.png | |||
| alt2 = Ball-and-stick model of the bithionol molecule | |||
| width2 = 240 | |||
== | <!--Clinical data--> | ||
| tradename = | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
== | <!--Identifiers--> | ||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 97-18-7 | |||
| ATC_prefix = D10 | |||
| ATC_suffix = AB01 | |||
| ATC_supplemental = {{ATC|P02|BX01}} {{ATCvet|P52|AG07}} | |||
| PubChem = 2406 | |||
| IUPHAR_ligand = 2338 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB04813 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = AMT77LS62O | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 290106 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 2313 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 3131 | |||
| smiles = Clc2cc(Cl)cc(Sc1cc(Cl)cc(Cl)c1O)c2O | |||
| InChI = 1/C12H6Cl4O2S/c13-5-1-7(15)11(17)9(3-5)19-10-4-6(14)2-8(16)12(10)18/h1-4,17-18H | |||
| InChIKey = JFIOVJDNOJYLKP-UHFFFAOYAO | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C12H6Cl4O2S/c13-5-1-7(15)11(17)9(3-5)19-10-4-6(14)2-8(16)12(10)18/h1-4,17-18H | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = JFIOVJDNOJYLKP-UHFFFAOYSA-N | |||
<!--Chemical data--> | |||
| C=12 | H=6 | Cl=4 | O=2 | S=1 | |||
| molecular_weight = 356.05 g/mol | |||
| synonyms = 2,4-dichloro- 6-(3,5-dichloro- 2-hydroxyphenyl)sulfanylphenol | |||
}} | |||
== | '''Bithionol''' is an [[anthelmintic]] used to treat ''[[Anoplocephala perfoliata]]'' (tapeworms) in horses<ref>{{ cite journal | author = Sanada Y, Senba H, Mochizuki R, Arakaki H, Gotoh T, Fukumoto S, Nagahata H | title = Evaluation of Marked Rise in Fecal Egg Output after Bithionol Administration to Horse and its Application as a Diagnostic Marker for Equine ''Anoplocephala perfoliata'' Infection | journal = Journal of Veterinary Medical Science | year = 2009 | volume = 71 | issue = 5 | pages = 617–620 | pmid = 19498288 | doi = 10.1292/jvms.71.617 | url = https://www.jstage.jst.go.jp/article/jvms/71/5/71_5_617/_pdf | format = pdf }}</ref> and ''[[Fasciola hepatica]]'' (liver flukes). | ||
==References== | |||
{{reflist}} | |||
{{Anthelmintics}} | |||
[[Category:Antiparasitic agents]] | |||
[[Category:Organochlorides]] | |||
[[Category:Phenols]] | |||
[[Category:Thioethers]] | |||
{{antiinfective-drug-stub}} | |||
{{dermatologic-drug-stub}} | |||
Revision as of 18:13, 13 April 2015
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | 2,4-dichloro- 6-(3,5-dichloro- 2-hydroxyphenyl)sulfanylphenol |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C12H6Cl4O2S |
| Molar mass | 356.05 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Bithionol is an anthelmintic used to treat Anoplocephala perfoliata (tapeworms) in horses[1] and Fasciola hepatica (liver flukes).
References
- ↑ Sanada Y, Senba H, Mochizuki R, Arakaki H, Gotoh T, Fukumoto S, Nagahata H (2009). "Evaluation of Marked Rise in Fecal Egg Output after Bithionol Administration to Horse and its Application as a Diagnostic Marker for Equine Anoplocephala perfoliata Infection" (pdf). Journal of Veterinary Medical Science. 71 (5): 617–620. doi:10.1292/jvms.71.617. PMID 19498288.
Template:Antiinfective-drug-stub
Template:Dermatologic-drug-stub
Categories:
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Antiparasitic agents
- Organochlorides
- Phenols
- Thioethers