Benzonatate: Difference between revisions
No edit summary |
No edit summary |
||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
|authorTag={{SS}} | |authorTag={{SS}} | ||
|genericName=Benzonatate | |genericName=Benzonatate | ||
|drugClass= | |aOrAn=an | ||
|drugClass=antitussive | |||
|indication=symptomatic relief of [[cough]] | |indication=symptomatic relief of [[cough]] | ||
|adverseReactions=[[ | |adverseReactions=[[nausea]], oral hypoesthesia, throat symptom, [[numbness]], [[dizziness]], [[headache]], [[sedation]], [[somnolence]] | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=<h4> | |fdaLIADAdult=<h4>Cough</h4> | ||
* Dosing information | * Dosing information | ||
:* Usual dose is: '''100 mg or 200 mg PO tid'''. | :* Usual dose is: '''100 mg or 200 mg PO tid'''. | ||
:* If necessary to control cough, up to '''600 mg PO tid''' divided doses may be given. | :* If necessary to control cough, up to '''600 mg PO tid''' divided doses may be given. | ||
:* | :* Benzonatate should be swallowed whole. Benzonatate Capsules are not to be broken, chewed, dissolved, cut or crushed. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of benzonatate in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of benzonatate in adult patients. | ||
|fdaLIADPed=<h4> | |fdaLIADPed=<h4>Cough</h4> | ||
* Dosing information (for children over 10 years of age) | * Dosing information (for children over 10 years of age) | ||
:* Usual dose is: '''100 mg or 200 mg PO tid'''. | :* Usual dose is: '''100 mg or 200 mg PO tid'''. | ||
:* If necessary to control cough, up to '''600 mg PO tid''' divided doses may be given. | :* If necessary to control cough, up to '''600 mg PO tid''' divided doses may be given. | ||
:* | :*Benzonatate should be swallowed whole. Benzonatate Capsules are not to be broken, chewed, dissolved, cut or crushed. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of benzonatate in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of benzonatate in pediatric patients. | ||
|contraindications=Hypersensitivity to benzonatate or related compounds. | |contraindications=[[Hypersensitivity]] to benzonatate or related compounds. | ||
|warnings=====Hypersensitivity==== | |warnings=====Hypersensitivity==== | ||
Severe hypersensitivity reactions (including bronchospasm, laryngospasm and cardiovascular collapse) have been reported which are possibly related to local anesthesia from sucking or chewing the capsule instead of swallowing it. Severe reactions have required intervention with vasopressor agents and supportive measures. | *Severe hypersensitivity reactions (including [[bronchospasm]], [[laryngospasm]] and cardiovascular collapse) have been reported which are possibly related to local anesthesia from sucking or chewing the capsule instead of swallowing it. Severe reactions have required intervention with vasopressor agents and supportive measures. | ||
====Psychiatric Effects==== | ====Psychiatric Effects==== | ||
Isolated instances of bizarre behavior, including mental confusion and visual | *Isolated instances of bizarre behavior, including mental confusion and visual [[hallucination]]s, have also been reported in patients taking benzonatate in combination with other prescribed drugs. | ||
====Accidental Ingestion and Death in Children==== | ====Accidental Ingestion and Death in Children==== | ||
Keep | *Keep benzonatate out of reach of children. Accidental ingestion of benzonatate resulting in death has been reported in children below age 10. Signs and symptoms of overdose have been reported within 15-20 minutes and death has been reported within one hour of ingestion. If accidental ingestion occurs, seek medical attention immediately | ||
|clinicalTrials=Potential Adverse Reactions to | |clinicalTrials=*Potential Adverse Reactions to Benzonatate may include: | ||
Hypersensitivity reactions including [[bronchospasm]], [[laryngospasm]], cardiovascular collapse possibly related to local [[anesthesia]] from chewing or sucking the capsule. | *[[Hypersensitivity]] reactions including [[bronchospasm]], [[laryngospasm]], cardiovascular collapse possibly related to local [[anesthesia]] from chewing or sucking the capsule. | ||
CNS: [[sedation]]; [[headache]]; [[dizziness]]; [[mental confusion]]; [[visual hallucinations]]. | *CNS: [[sedation]]; [[headache]]; [[dizziness]]; [[mental confusion]]; [[visual hallucinations]]. | ||
GI: [[constipation]]; [[nausea]]; GI upset. | *GI: [[constipation]]; [[nausea]]; GI upset. | ||
Dermatologic: [[pruritus]]; [[skin eruptions]]. | *Dermatologic: [[pruritus]]; [[skin eruptions]]. | ||
Other: nasal congestion; sensation of burning in the eyes; vague “chilly” sensation; numbness of the chest; hypersensitivity. | *Other: [[nasal congestion]]; sensation of burning in the eyes; vague “chilly” sensation; numbness of the chest; [[hypersensitivity]]. | ||
Deliberate or accidental overdose has resulted in death, particularly in children. | *Deliberate or accidental overdose has resulted in death, particularly in children. | ||
|postmarketing=FDA package insert for benzonatate contains no information regarding postmarkting experience. | |||
|postmarketing=FDA | |drugInteractions=FDA Package Insert for benzonatate contains no information regarding drug interaction. | ||
|drugInteractions=FDA Package Insert for | |||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=Animal reproduction studies have not been conducted with | |useInPregnancyFDA=Animal reproduction studies have not been conducted with benzonatate. It is also not known whether benzonatate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzonatate should be given to a pregnant woman only if clearly needed. | ||
|useInNursing=It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk caution should be exercised when | |useInNursing=It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk caution should be exercised when benzonatate is administered to a nursing woman. | ||
|useInGeri=Safety and effectiveness in children below the age of 10 have not been established. Accidental ingestion resulting in death has been reported in children below age 10. Keep out of reach of children. | |useInGeri=Safety and effectiveness in children below the age of 10 have not been established. Accidental ingestion resulting in death has been reported in children below age 10. Keep out of reach of children. | ||
|administration= | |administration=Benzonatate should be swallowed whole. Benzonatate capsules are not to be broken, chewed, dissolved, cut or crushed. | ||
|monitoring=FDA | |monitoring=FDA package insert for benzonatate contains no information regarding drug monitoring. | ||
|IVCompat=There is limited information about the IV | |IVCompat=There is limited information about the IV compatibility. | ||
|overdose=Intentional and unintentional overdose may result in death, particularly in children. | |overdose=*Intentional and unintentional overdose may result in death, particularly in children. | ||
The drug is chemically related to tetracaine and other topical anesthetics and shares various aspects of their pharmacology and toxicology. Drugs of this type are generally well absorbed after ingestion. | *The drug is chemically related to [[tetracaine]] and other topical anesthetics and shares various aspects of their pharmacology and toxicology. Drugs of this type are generally well absorbed after ingestion. | ||
'''Signs and Symptoms:''' | '''Signs and Symptoms:''' | ||
The signs and symptoms of overdose of benzonatate have been reported within 15-20 minutes. If capsules are chewed or dissolved in the mouth, oropharyngeal anesthesia will develop rapidly, which may cause choking and airway compromise. | *The signs and symptoms of overdose of benzonatate have been reported within 15-20 minutes. If capsules are chewed or dissolved in the mouth, oropharyngeal anesthesia will develop rapidly, which may cause choking and airway compromise. | ||
CNS stimulation may cause restlessness and | *CNS stimulation may cause restlessness and [[tremor]]s which may proceed to clonic [[convulsions]] followed by profound CNS [[depression]]. [[Convulsions]], [[coma]], [[cerebral edema]] and [[cardiac arrest]] leading to death have been reported within 1 hour of ingestion. | ||
'''Treatment:''' | '''Treatment:''' | ||
In case of overdose, seek medical attention immediately. Evacuate gastric contents and administer copious amounts of activated charcoal slurry. Even in the conscious patient, cough and gag reflexes may be so depressed as to necessitate special attention to protection against aspiration of gastric contents and orally administered materials. Convulsions should be treated with a short-acting barbiturate given intravenously and carefully titrated for the smallest effective dosage. Intensive support of respiration and cardiovascular-renal function is an essential feature of the treatment of severe intoxication from overdosage. | *In case of overdose, seek medical attention immediately. Evacuate gastric contents and administer copious amounts of activated charcoal slurry. Even in the conscious patient, cough and gag reflexes may be so depressed as to necessitate special attention to protection against aspiration of gastric contents and orally administered materials. [[Convulsions]] should be treated with a short-acting barbiturate given intravenously and carefully titrated for the smallest effective dosage. Intensive support of respiration and cardiovascular-renal function is an essential feature of the treatment of severe intoxication from overdosage. | ||
Do not use CNS stimulants. | *Do not use CNS stimulants. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 127: | Line 127: | ||
| StdInChIKey = MAFMQEKGGFWBAB-UHFFFAOYSA-N | | StdInChIKey = MAFMQEKGGFWBAB-UHFFFAOYSA-N | ||
}} | }} | ||
|mechAction= | |mechAction=*Benzonatate acts peripherally by anesthetizing the stretch receptors located in the respiratory passages, lungs, and pleura by dampening their activity and thereby reducing the cough reflex at its source. It begins to act within 15 to 20 minutes and its effect lasts for 3 to 8 hours. Benzonatate has no inhibitory effect on the respiratory center in recommended dosage. | ||
|structure=Benzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p(butylamino) benzoate; with a molecular weight of 603.7. | |structure=*Benzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p(butylamino) benzoate; with a molecular weight of 603.7. | ||
[[File:Benzonatate_structure_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Benzonatate_structure_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
Each soft gelatin capsule, for oral administration, contains 100 mg or 200 mg benzonatate USP. In addition, each capsule contains the following inactive ingredients: D&C Yellow No. 10, gelatin, glycerin, and purified water. | *Each soft gelatin capsule, for oral administration, contains 100 mg or 200 mg benzonatate USP. In addition, each capsule contains the following inactive ingredients: D&C Yellow No. 10, gelatin, glycerin, and purified water. | ||
|nonClinToxic=Carcinogenicity, mutagenicity, and reproduction studies have not been conducted with Benzonatate. | |||
|nonClinToxic=Carcinogenicity, mutagenicity, and reproduction studies have not been conducted with | |||
|clinicalStudies=There is limited information about the clinical studies. | |clinicalStudies=There is limited information about the clinical studies. | ||
|howSupplied=Benzonatate Capsules USP, 100 mg: Yellow soft gelatin capsules, imprinted with “PA46”, available in bottles of 100’s and 500’s | |howSupplied=*Benzonatate Capsules USP, 100 mg: Yellow soft gelatin capsules, imprinted with “PA46”, available in bottles of 100’s and 500’s. | ||
|fdaPatientInfo=Swallow | *Benzonatate Capsules USP, 200 mg: Yellow soft gelatin capsules, imprinted with “PA83”, available in bottles of 100’s. | ||
|storage=*The capsules should be protected from light, moisture and humidity, and stored at controlled room temperature 20° to 25°C (68° to 77°F) [See USP]. | |||
|fdaPatientInfo=*Swallow benzonatate capsules whole. Do not break, chew, dissolve, cut, or crush benzonatate capsules. Release of benzonatate from the capsule in the mouth can produce a temporary local anesthesia of the oral mucosa and choking could occur. If numbness or tingling of the tongue, mouth, throat, or face occurs, refrain from oral ingestion of food or liquids until the numbness has resolved. If the symptoms worsen or persist, seek medical attention. | |||
Keep | *Keep benzonatate out of reach of children. Accidental ingestion resulting in death has been reported in children. Signs and symptoms of overdose have been reported within 15-20 minutes and death has been reported within one hour of ingestion. Signs and symptoms may include restlessness, [[tremor]]s, [[convulsions]], [[coma]] and [[cardiac arrest]]. If accidental ingestion occurs, seek medical attention immediately. | ||
Overdosage resulting in death may occur in adults. | *Overdosage resulting in death may occur in adults. | ||
Do not exceed a single dose of 200 mg and a total daily dosage of 600 mg. If you miss a dose of | *Do not exceed a single dose of 200 mg and a total daily dosage of 600 mg. If you miss a dose of benzonatate, skip that dose and take the next dose at the next scheduled time. Do not take 2 doses of benzonatate at one time. | ||
|alcohol=Alcohol-Benzonatate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Benzonatate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* Tessalon Perles | |brandNames=* Tessalon Perles | ||
| Line 153: | Line 151: | ||
|lookAlike=There is limited information about the look-alike drug names. | |lookAlike=There is limited information about the look-alike drug names. | ||
}} | }} | ||
{{PillImage | {{PillImage|fileName=Benzonatate_NDC_05551883.jpg|drugName=Benzonatate|NDC=05551883|drugAuthor=Barr Laboratories Inc.|ingredients=BENZONATATE[BENZONATATE]|pillImprint=PA83|dosageValue=200|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=12|pillScore=1}} | ||
|fileName= | {{PillImage|fileName=benzonatate_NDC_09045904.jpg|drugName=benzonatate|NDC=09045904|drugAuthor=MAJOR PHARMACEUTICALS INC|ingredients=benzonatate[benzonatate]|pillImprint=ASC;105|dosageValue=100|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=19|pillScore=1}} | ||
|drugName=Benzonatate 100 | {{PillImage|fileName=Benzonatate_NDC_501110851.jpg|drugName=Benzonatate|NDC=501110851|drugAuthor=Pliva Inc.|ingredients=BENZONATATE[BENZONATATE]|pillImprint=PA46|dosageValue=100|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=9|pillScore=1}} | ||
|NDC= | {{PillImage|fileName=BENZONATATE_NDC_576640133.jpg|drugName=BENZONATATE|NDC=576640133|drugAuthor=Caraco Pharmaceutical Laboratories, Ltd.|ingredients=BENZONATATE[BENZONATATE]|pillImprint=133|dosageValue=100|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=3|pillScore=1}} | ||
|drugAuthor= | {{PillImage|fileName=Benzonatate_NDC_604290018.jpg|drugName=Benzonatate|NDC=604290018|drugAuthor=Golden State Medical Supply, Inc.|ingredients=benzonatate[benzonatate]|pillImprint=ASC;105|dosageValue=100|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=19|pillScore=1}} | ||
| | {{PillImage|fileName=Benzonatate_NDC_604290028.jpg|drugName=Benzonatate|NDC=604290028|drugAuthor=Golden State Medical Supply, Inc.|ingredients=benzonatate[benzonatate]|pillImprint=ASC;106|dosageValue=100|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=19|pillScore=1}} | ||
|pillImprint= | {{PillImage|fileName=Benzonatate_NDC_651620536.jpg|drugName=Benzonatate|NDC=651620536|drugAuthor=Amneal Pharmaceuticals of NY LLC|ingredients=BENZONATATE[BENZONATATE]|pillImprint=A1|dosageValue=100|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=9|pillScore=1}} | ||
|dosageValue=100 | {{PillImage|fileName=Benzonatate_NDC_651620537.jpg|drugName=Benzonatate|NDC=651620537|drugAuthor=Amneal Pharmaceuticals of NY LLC|ingredients=BENZONATATE[BENZONATATE]|pillImprint=A2|dosageValue=200|dosageUnit=mg|pillColor=Yellow|pillShape=Oval|pillSize=16|pillScore=1}} | ||
|dosageUnit=mg | |||
|pillColor=Yellow | |||
|pillShape=Oval | |||
|pillSize=9. | |||
|pillScore=1 | |||
}} | |||
{{LabelImage | {{LabelImage | ||
|fileName=Benzonatate_label_01.jpg | |fileName=Benzonatate_label_01.jpg | ||
Latest revision as of 15:05, 8 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Benzonatate is an antitussive that is FDA approved for the {{{indicationType}}} of symptomatic relief of cough. Common adverse reactions include nausea, oral hypoesthesia, throat symptom, numbness, dizziness, headache, sedation, somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Cough
- Dosing information
- Usual dose is: 100 mg or 200 mg PO tid.
- If necessary to control cough, up to 600 mg PO tid divided doses may be given.
- Benzonatate should be swallowed whole. Benzonatate Capsules are not to be broken, chewed, dissolved, cut or crushed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of benzonatate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of benzonatate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Cough
- Dosing information (for children over 10 years of age)
- Usual dose is: 100 mg or 200 mg PO tid.
- If necessary to control cough, up to 600 mg PO tid divided doses may be given.
- Benzonatate should be swallowed whole. Benzonatate Capsules are not to be broken, chewed, dissolved, cut or crushed.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of benzonatate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of benzonatate in pediatric patients.
Contraindications
Hypersensitivity to benzonatate or related compounds.
Warnings
Hypersensitivity
- Severe hypersensitivity reactions (including bronchospasm, laryngospasm and cardiovascular collapse) have been reported which are possibly related to local anesthesia from sucking or chewing the capsule instead of swallowing it. Severe reactions have required intervention with vasopressor agents and supportive measures.
Psychiatric Effects
- Isolated instances of bizarre behavior, including mental confusion and visual hallucinations, have also been reported in patients taking benzonatate in combination with other prescribed drugs.
Accidental Ingestion and Death in Children
- Keep benzonatate out of reach of children. Accidental ingestion of benzonatate resulting in death has been reported in children below age 10. Signs and symptoms of overdose have been reported within 15-20 minutes and death has been reported within one hour of ingestion. If accidental ingestion occurs, seek medical attention immediately
Adverse Reactions
Clinical Trials Experience
- Potential Adverse Reactions to Benzonatate may include:
- Hypersensitivity reactions including bronchospasm, laryngospasm, cardiovascular collapse possibly related to local anesthesia from chewing or sucking the capsule.
- GI: constipation; nausea; GI upset.
- Dermatologic: pruritus; skin eruptions.
- Other: nasal congestion; sensation of burning in the eyes; vague “chilly” sensation; numbness of the chest; hypersensitivity.
- Deliberate or accidental overdose has resulted in death, particularly in children.
Postmarketing Experience
FDA package insert for benzonatate contains no information regarding postmarkting experience.
Drug Interactions
FDA Package Insert for benzonatate contains no information regarding drug interaction.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been conducted with benzonatate. It is also not known whether benzonatate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzonatate should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Benzonatate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Benzonatate during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk caution should be exercised when benzonatate is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Benzonatate in pediatric settings.
Geriatic Use
Safety and effectiveness in children below the age of 10 have not been established. Accidental ingestion resulting in death has been reported in children below age 10. Keep out of reach of children.
Gender
There is no FDA guidance on the use of Benzonatate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Benzonatate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Benzonatate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Benzonatate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Benzonatate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Benzonatate in patients who are immunocompromised.
Administration and Monitoring
Administration
Benzonatate should be swallowed whole. Benzonatate capsules are not to be broken, chewed, dissolved, cut or crushed.
Monitoring
FDA package insert for benzonatate contains no information regarding drug monitoring.
IV Compatibility
There is limited information about the IV compatibility.
Overdosage
- Intentional and unintentional overdose may result in death, particularly in children.
- The drug is chemically related to tetracaine and other topical anesthetics and shares various aspects of their pharmacology and toxicology. Drugs of this type are generally well absorbed after ingestion.
Signs and Symptoms:
- The signs and symptoms of overdose of benzonatate have been reported within 15-20 minutes. If capsules are chewed or dissolved in the mouth, oropharyngeal anesthesia will develop rapidly, which may cause choking and airway compromise.
- CNS stimulation may cause restlessness and tremors which may proceed to clonic convulsions followed by profound CNS depression. Convulsions, coma, cerebral edema and cardiac arrest leading to death have been reported within 1 hour of ingestion.
Treatment:
- In case of overdose, seek medical attention immediately. Evacuate gastric contents and administer copious amounts of activated charcoal slurry. Even in the conscious patient, cough and gag reflexes may be so depressed as to necessitate special attention to protection against aspiration of gastric contents and orally administered materials. Convulsions should be treated with a short-acting barbiturate given intravenously and carefully titrated for the smallest effective dosage. Intensive support of respiration and cardiovascular-renal function is an essential feature of the treatment of severe intoxication from overdosage.
- Do not use CNS stimulants.
Pharmacology
| |
Benzonatate
| |
| Systematic (IUPAC) name | |
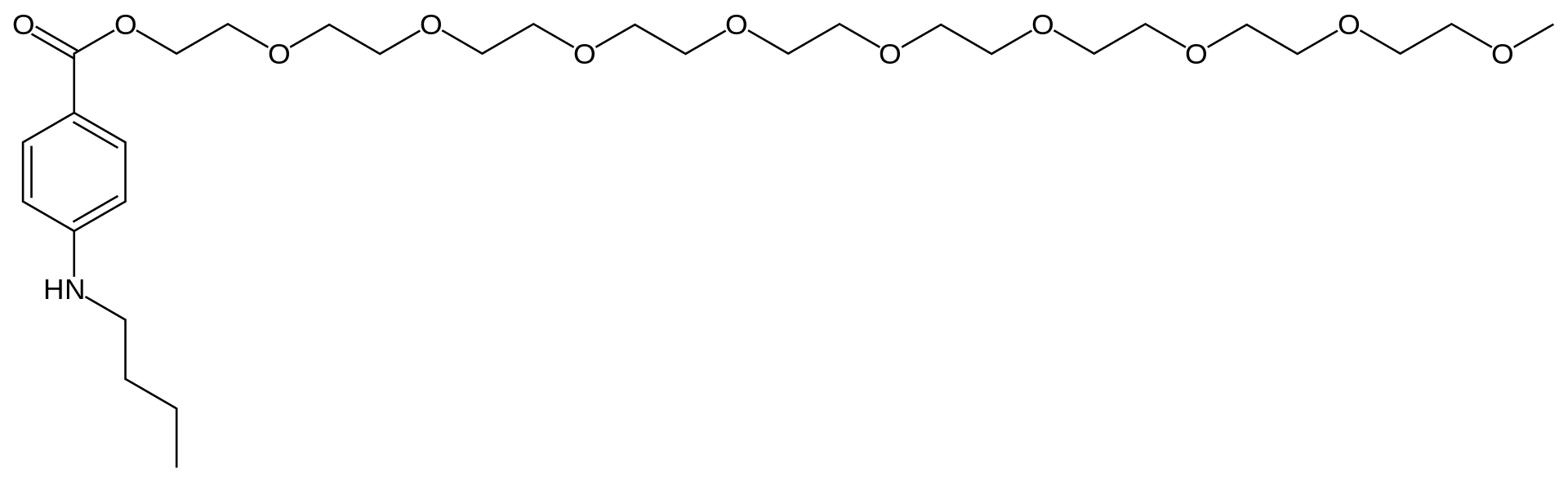
| 2-[2-[2-[2-[2-[2-[2-[2- (2-methoxyethoxy) ethoxy] ethoxy] ethoxy] ethoxy] ethoxy] ethoxy] ethoxy] ethyl4-butylaminobenzoate | |
| Identifiers | |
| CAS number | |
| ATC code | R05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 603.742 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 3-8 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Benzonatate acts peripherally by anesthetizing the stretch receptors located in the respiratory passages, lungs, and pleura by dampening their activity and thereby reducing the cough reflex at its source. It begins to act within 15 to 20 minutes and its effect lasts for 3 to 8 hours. Benzonatate has no inhibitory effect on the respiratory center in recommended dosage.
Structure
- Benzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p(butylamino) benzoate; with a molecular weight of 603.7.

- Each soft gelatin capsule, for oral administration, contains 100 mg or 200 mg benzonatate USP. In addition, each capsule contains the following inactive ingredients: D&C Yellow No. 10, gelatin, glycerin, and purified water.
Pharmacodynamics
There is limited information regarding Benzonatate Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Benzonatate Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenicity, mutagenicity, and reproduction studies have not been conducted with Benzonatate.
Clinical Studies
There is limited information about the clinical studies.
How Supplied
- Benzonatate Capsules USP, 100 mg: Yellow soft gelatin capsules, imprinted with “PA46”, available in bottles of 100’s and 500’s.
- Benzonatate Capsules USP, 200 mg: Yellow soft gelatin capsules, imprinted with “PA83”, available in bottles of 100’s.
Storage
- The capsules should be protected from light, moisture and humidity, and stored at controlled room temperature 20° to 25°C (68° to 77°F) [See USP].
Images
Drug Images
{{#ask: Page Name::Benzonatate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Benzonatate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Swallow benzonatate capsules whole. Do not break, chew, dissolve, cut, or crush benzonatate capsules. Release of benzonatate from the capsule in the mouth can produce a temporary local anesthesia of the oral mucosa and choking could occur. If numbness or tingling of the tongue, mouth, throat, or face occurs, refrain from oral ingestion of food or liquids until the numbness has resolved. If the symptoms worsen or persist, seek medical attention.
- Keep benzonatate out of reach of children. Accidental ingestion resulting in death has been reported in children. Signs and symptoms of overdose have been reported within 15-20 minutes and death has been reported within one hour of ingestion. Signs and symptoms may include restlessness, tremors, convulsions, coma and cardiac arrest. If accidental ingestion occurs, seek medical attention immediately.
- Overdosage resulting in death may occur in adults.
- Do not exceed a single dose of 200 mg and a total daily dosage of 600 mg. If you miss a dose of benzonatate, skip that dose and take the next dose at the next scheduled time. Do not take 2 doses of benzonatate at one time.
Precautions with Alcohol
Alcohol-Benzonatate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Tessalon Perles
- Zonatuss
Look-Alike Drug Names
There is limited information about the look-alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Benzonatate |Pill Name=Benzonatate_NDC_05551883.jpg |Drug Name=Benzonatate |Pill Ingred=BENZONATATE[BENZONATATE]|+sep=; |Pill Imprint=PA83 |Pill Dosage=200 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=12 |Pill Scoring=1 |Pill Image= |Drug Author=Barr Laboratories Inc. |NDC=05551883
}}
{{#subobject:
|Page Name=Benzonatate |Pill Name=benzonatate_NDC_09045904.jpg |Drug Name=benzonatate |Pill Ingred=benzonatate[benzonatate]|+sep=; |Pill Imprint=ASC;105 |Pill Dosage=100 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=MAJOR PHARMACEUTICALS INC |NDC=09045904
}}
{{#subobject:
|Page Name=Benzonatate |Pill Name=Benzonatate_NDC_501110851.jpg |Drug Name=Benzonatate |Pill Ingred=BENZONATATE[BENZONATATE]|+sep=; |Pill Imprint=PA46 |Pill Dosage=100 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Pliva Inc. |NDC=501110851
}}
{{#subobject:
|Page Name=Benzonatate |Pill Name=BENZONATATE_NDC_576640133.jpg |Drug Name=BENZONATATE |Pill Ingred=BENZONATATE[BENZONATATE]|+sep=; |Pill Imprint=133 |Pill Dosage=100 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=3 |Pill Scoring=1 |Pill Image= |Drug Author=Caraco Pharmaceutical Laboratories, Ltd. |NDC=576640133
}}
{{#subobject:
|Page Name=Benzonatate |Pill Name=Benzonatate_NDC_604290018.jpg |Drug Name=Benzonatate |Pill Ingred=benzonatate[benzonatate]|+sep=; |Pill Imprint=ASC;105 |Pill Dosage=100 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290018
}}
{{#subobject:
|Page Name=Benzonatate |Pill Name=Benzonatate_NDC_604290028.jpg |Drug Name=Benzonatate |Pill Ingred=benzonatate[benzonatate]|+sep=; |Pill Imprint=ASC;106 |Pill Dosage=100 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290028
}}
{{#subobject:
|Page Name=Benzonatate |Pill Name=Benzonatate_NDC_651620536.jpg |Drug Name=Benzonatate |Pill Ingred=BENZONATATE[BENZONATATE]|+sep=; |Pill Imprint=A1 |Pill Dosage=100 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=9 |Pill Scoring=1 |Pill Image= |Drug Author=Amneal Pharmaceuticals of NY LLC |NDC=651620536
}}
{{#subobject:
|Page Name=Benzonatate |Pill Name=Benzonatate_NDC_651620537.jpg |Drug Name=Benzonatate |Pill Ingred=BENZONATATE[BENZONATATE]|+sep=; |Pill Imprint=A2 |Pill Dosage=200 mg |Pill Color=Yellow|+sep=; |Pill Shape=Oval |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=Amneal Pharmaceuticals of NY LLC |NDC=651620537
}}
{{#subobject:
|Label Page=Benzonatate |Label Name=Benzonatate_label_01.jpg
}}
{{#subobject:
|Label Page=Benzonatate |Label Name=Benzonatate_panel_01.png
}}