Benzethonium chloride: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
Rabin Bista (talk | contribs) No edit summary |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag=<!--Overview--> | |authorTag=<!--Overview-->{{RB}} | ||
|OTC=Yes | |||
|genericName=Benzethonium chloride and benzocaine | |||
|aOrAn=a | |aOrAn=a | ||
| | |drugClass=OTC antiseptic | ||
|adverseReactions=<!--Black Box Warning--> | |indicationType=treatment | ||
|indication=first aid for the temporary relief of [[pain]] and [[itching]] and to help prevent infection in [[minor cuts]], [[scrapes]] and [[burns]] | |||
|adverseReactions=[[Dry skin]], [[redness]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult====== | |fdaLIADAdult=====Indications==== | ||
* first aid for the temporary relief of pain and itching and to help prevent infection in [[minor cuts]], [[scrapes]] and [[burns]] | |||
* | |||
====Directions==== | |||
:* | * adults and children 2 years of age and older: clean the affected area and apply a small amount to the affected area not more than 1 to 3 times daily | ||
* children under 2 years of age: ask a doctor | |||
= | * may be covered with a sterile bandage | ||
|offLabelAdultGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
* | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=* There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
* | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
* | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
* | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications | |contraindications | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings======For external use only===== | ||
==== | |||
* Do not use | |||
:* in the eyes | |||
:* over large areas of the body | |||
===== | =====Ask a doctor before use if you have===== | ||
:* deep or [[puncture wounds]] | |||
:* [[animal bites]] | |||
:* serious [[burns]] | |||
=====Stop use and ask a doctor if===== | |||
:* condition worsens | |||
:* needed for longer than 1 week | |||
* Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
= | |clinical trial Experience=* [[dry skin]], [[redness]] | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=* There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions= | |drugInteractions= | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
| Line 264: | Line 102: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration= | |administration=====Directions==== | ||
* | * adults and children 2 years of age and older: clean the affected area and apply a small amount to the affected area not more than 1 to 3 times daily | ||
* children under 2 years of age: ask a doctor | |||
* may be covered with a sterile bandage | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 275: | Line 115: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=* There is limited information regarding <i>Overdose</i> of {{PAGENAME}} in the drug label. | ||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox=: [[File:Benzethonium chloride wiki chembox.png|thumb|none|500px|This image is privided by Wikipedia]] | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
<!--Structure--> | |||
|structure=====Active Ingredients==== | |||
<!-- | |||
: [[File:Benzethonium chloride act ing.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
| | |||
====Inactive ingredients==== | |||
acetylated lanolin alcohol, aloe, cetyl acetate, cetyl alcohol, cholecalciferol, corn oil, dimethicone, dl-alpha tocopherol acetate, fragrance, glycerin, glyceryl monostearate, isopropyl myristate, methylparaben, mineral oil, PEG-100 stearate, polyvinylpyrrolidone/eicosene copolymer, propylparaben, pyrithione zinc, retinyl palmitate, sorbitan monostearate, stearamidopropyl PG-dimonium chloride phosphate, water | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 313: | Line 149: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied= | ||
|packLabel= | |storage=* store at 20-25°C (68-77°F) | ||
* retain carton for future reference | |||
|packLabel=====PRINCIPAL DISPLAY PANEL - 28 g Tube Carton==== | |||
NDC 63824-810-01 | |||
LANACANE® | |||
Maximum Strength | |||
ANTI-ITCH | |||
CREAM | |||
Benzethonium chloride 0.2% | |||
(First-Aid Antiseptic) | |||
Benzocaine 20% | |||
(Pain Relief Cream) | |||
<!-- | 2-in-1 | ||
FAST Acting | |||
Itch Relief | |||
+ | |||
Kills Germs* | |||
*unlike Hydrocortisones | |||
Itch Relief from: | |||
Insect bites | |||
Rashes | |||
Dry, Itchy skin | |||
NET WT 1 OZ (28 g) | |||
: [[File:Benzethonium chloride PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Ingredients and Appearance==== | |||
: [[File:Benzethonium chloride Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=* Questions? | |||
: 1-866-252-5327 FREE. | |||
* You may also report side effects to this phone number. | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* LANACANE MAXIMUM STRENGTH ANTI-ITCH®<ref>{{Cite web | title = benzethonium chloride and benzocaine cream | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=96eb2dcc-4653-452a-9abd-fe92114a14ad}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
Latest revision as of 18:36, 26 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Benzethonium chloride is a OTC antiseptic that is FDA approved for the treatment of first aid for the temporary relief of pain and itching and to help prevent infection in minor cuts, scrapes and burns. Common adverse reactions include Dry skin, redness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- first aid for the temporary relief of pain and itching and to help prevent infection in minor cuts, scrapes and burns
Directions
- adults and children 2 years of age and older: clean the affected area and apply a small amount to the affected area not more than 1 to 3 times daily
- children under 2 years of age: ask a doctor
- may be covered with a sterile bandage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Benzethonium chloride in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Benzethonium chloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- There is limited information regarding FDA-Labeled Use of Benzethonium chloride in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Benzethonium chloride in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Benzethonium chloride in pediatric patients.
Contraindications
There is limited information regarding Benzethonium chloride Contraindications in the drug label.
Warnings
For external use only
- Do not use
- in the eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens
- needed for longer than 1 week
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Benzethonium chloride Clinical Trials Experience in the drug label.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Benzethonium chloride in the drug label.
Drug Interactions
There is limited information regarding Benzethonium chloride Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Benzethonium chloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Benzethonium chloride during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Benzethonium chloride with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Benzethonium chloride with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Benzethonium chloride with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Benzethonium chloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Benzethonium chloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Benzethonium chloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Benzethonium chloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Benzethonium chloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Benzethonium chloride in patients who are immunocompromised.
Administration and Monitoring
Administration
Directions
- adults and children 2 years of age and older: clean the affected area and apply a small amount to the affected area not more than 1 to 3 times daily
- children under 2 years of age: ask a doctor
- may be covered with a sterile bandage
Monitoring
There is limited information regarding Monitoring of Benzethonium chloride in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Benzethonium chloride in the drug label.
Overdosage
- There is limited information regarding Overdose of Benzethonium chloride in the drug label.
Pharmacology
Mechanism of Action
There is limited information regarding Benzethonium chloride Mechanism of Action in the drug label.
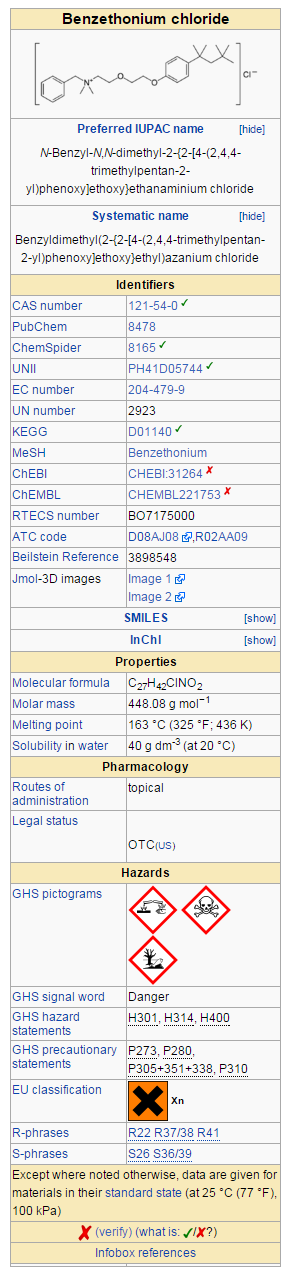
Structure
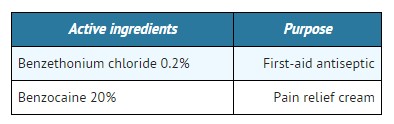
Active Ingredients
Inactive ingredients
acetylated lanolin alcohol, aloe, cetyl acetate, cetyl alcohol, cholecalciferol, corn oil, dimethicone, dl-alpha tocopherol acetate, fragrance, glycerin, glyceryl monostearate, isopropyl myristate, methylparaben, mineral oil, PEG-100 stearate, polyvinylpyrrolidone/eicosene copolymer, propylparaben, pyrithione zinc, retinyl palmitate, sorbitan monostearate, stearamidopropyl PG-dimonium chloride phosphate, water
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Benzethonium chloride in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Benzethonium chloride in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Benzethonium chloride in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Benzethonium chloride in the drug label.
How Supplied
There is limited information regarding Benzethonium chloride How Supplied in the drug label.
Storage
- store at 20-25°C (68-77°F)
- retain carton for future reference
Images
Drug Images
{{#ask: Page Name::Benzethonium chloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
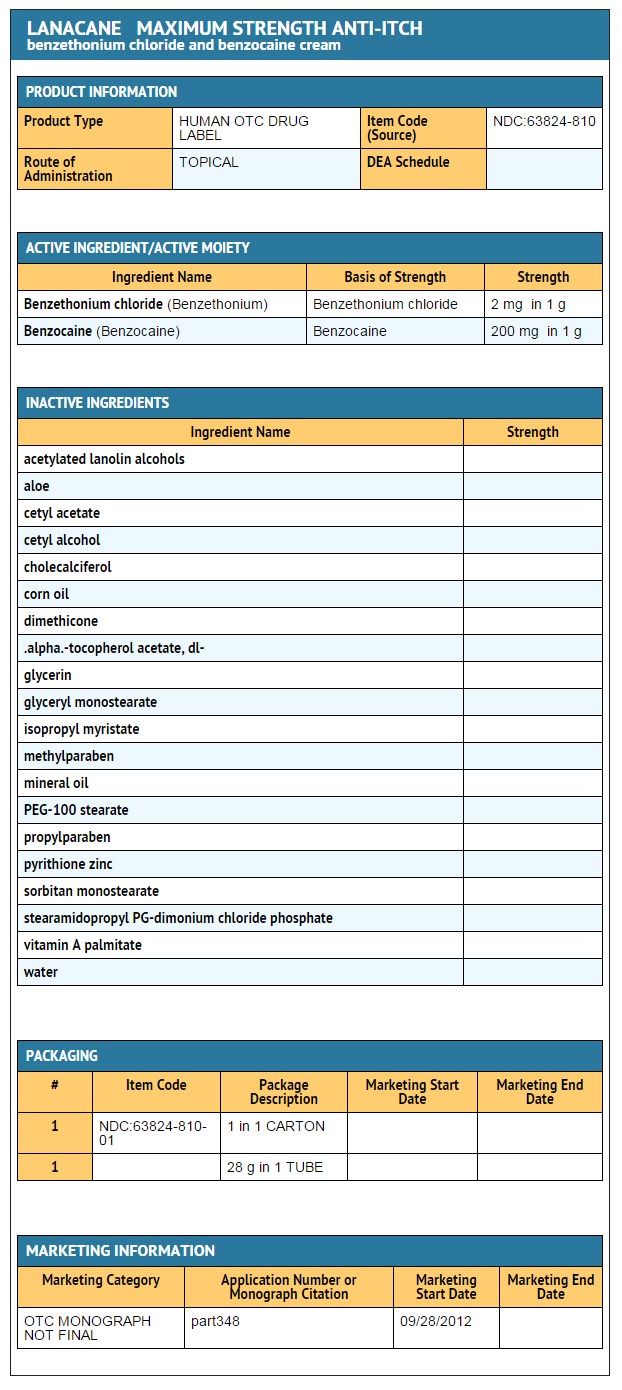
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 28 g Tube Carton
NDC 63824-810-01
LANACANE®
Maximum Strength
ANTI-ITCH CREAM
Benzethonium chloride 0.2% (First-Aid Antiseptic) Benzocaine 20% (Pain Relief Cream)
2-in-1
FAST Acting Itch Relief
+
Kills Germs*
- unlike Hydrocortisones
Itch Relief from:
Insect bites Rashes Dry, Itchy skin
NET WT 1 OZ (28 g)
Ingredients and Appearance
{{#ask: Label Page::Benzethonium chloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Questions?
- 1-866-252-5327 FREE.
- You may also report side effects to this phone number.
Precautions with Alcohol
- Alcohol-Benzethonium chloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LANACANE MAXIMUM STRENGTH ANTI-ITCH®[1]
Look-Alike Drug Names
There is limited information regarding Benzethonium chloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.