Belatacept: Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
Kiran Singh (talk | contribs) No edit summary |
||
| Line 43: | Line 43: | ||
:*The nearest dose to 640 mg is 637.5 mg. | :*The nearest dose to 640 mg is 637.5 mg. | ||
:*Therefore, the actual prescribed dose for the patient should be 637.5 mg. | :*Therefore, the actual prescribed dose for the patient should be 637.5 mg. | ||
[[File:Belatacept table1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
| Line 82: | Line 65: | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
|administration=* Intravenous | |||
'''Preparation and Administration Instructions''' | |||
* NULOJIX is for intravenous infusion only. | |||
* ''Caution'': NULOJIX must be reconstituted/prepared using only the silicone-free disposable syringe provided with each vial. | |||
* If the silicone-free disposable syringe is dropped or becomes contaminated, use a new silicone-free disposable syringe from inventory. For information on obtaining additional silicone-free disposable syringes, contact Bristol-Myers Squibb at 1-888-NULOJIX. | |||
'''Preparation for Administration''' | |||
* Calculate the number of NULOJIX vials required to provide the total infusion dose. Each vial contains 250 mg of belatacept lyophilized powder. | |||
* Reconstitute the contents of each vial of NULOJIX with 10.5 mL of a suitable diluent using the silicone-free disposable syringe provided with each vial and an 18- to 21-gauge needle. Suitable diluents include: sterile water for injection (SWFI), 0.9% sodium chloride (NS), or 5% dextrose in water (D5W). | |||
Note: If the NULOJIX powder is accidentally reconstituted using a different syringe than the one provided, the solution may develop a few translucent particles. Discard any solutions prepared using siliconized syringes. | |||
* To reconstitute the NULOJIX powder, remove the flip-top from the vial and wipe the top with an alcohol swab. Insert the syringe needle into the vial through the center of the rubber stopper and direct the stream of diluent (10.5 mL of SWFI, NS, or D5W) to the glass wall of the vial. | |||
* To minimize foam formation, rotate the vial and invert with gentle swirling until the contents are completely dissolved. Avoid prolonged or vigorous agitation. Do not shake. | |||
* The reconstituted solution contains a belatacept concentration of 25 mg/mL and should be clear to slightly opalescent and colorless to pale yellow. Do not use if opaque particles, discoloration, or other foreign particles are present. | |||
* Calculate the total volume of the reconstituted 25 mg/mL NULOJIX solution required to provide the total infusion dose. | |||
Volume of 25 mg/mL NULOJIX solution (in mL) = Prescribed Dose (in mg) ÷ 25 mg/mL | |||
* Prior to intravenous infusion, the required volume of the reconstituted NULOJIX solution must be further diluted with a suitable infusion fluid (NS or D5W). NULOJIX reconstituted with: | |||
:* SWFI should be further diluted with either NS or D5W | |||
:* NS should be further diluted with NS | |||
:*D5W should be further diluted with D5W | |||
* From the appropriate size infusion bag or bottle, withdraw a volume of infusion fluid that is equal to the volume of the reconstituted NULOJIX solution required to provide the prescribed dose. With the same silicone-free disposable syringe used for reconstitution, withdraw the required amount of belatacept solution from the vial, inject it into the infusion bag or bottle, and gently rotate the infusion bag or bottle to ensure mixing. | |||
The final belatacept concentration in the infusion bag or bottle should range from 2 mg/mL to 10 mg/mL. Typically, an infusion volume of 100 mL will be appropriate for most patients and doses, but total infusion volumes ranging from 50 mL to 250 mL may be used. Any unused solution remaining in the vials must be discarded. | |||
* Prior to administration, the NULOJIX infusion should be inspected visually for particulate matter and discoloration. Discard the infusion if any particulate matter or discoloration is observed. | |||
* The entire NULOJIX infusion should be administered over a period of 30 minutes and must be administered with an infusion set and a sterile, non-pyrogenic, low-protein-binding filter (with a pore size of 0.2-1.2 µm). | |||
:*The reconstituted solution should be transferred from the vial to the infusion bag or bottle immediately. The NULOJIX infusion must be completed within 24 hours of reconstitution of the NULOJIX lyophilized powder. If not used immediately, the infusion solution may be stored under refrigeration conditions: 2°-8°C (36°-46°F) and protected from light for up to 24 hours (a maximum of 4 hours of the total 24 hours can be at room temperature: 20°-25°C [68°-77°F] and room light). | |||
:* Infuse NULOJIX in a separate line from other concomitantly infused agents. NULOJIX should not be infused concomitantly in the same intravenous line with other agents. No physical or biochemical compatibility studies have been conducted to evaluate the coadministration of NULOJIX with other agents. | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|overdose=There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug box 2--> | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | ||
|howSupplied=* | |howSupplied=* | ||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* NULOJIX | |brandNames=* NULOJIX | ||
|lookAlike=* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |lookAlike=* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | ||
Revision as of 17:58, 12 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: POST-TRANSPLANT LYMPHOPROLIFERATIVE DISORDER, OTHER MALIGNANCIES, AND SERIOUS INFECTIONS
See full prescribing information for complete Boxed Warning.
* Increased risk for developing post-transplant lymphoproliferative disorder (PTLD), predominantly involving the central nervous system (CNS). Recipients without immunity to Epstein-Barr virus (EBV) are at a particularly increased risk; therefore, use in EBV seropositive patients only. Do not use NULOJIX in transplant recipients who are EBV seronegative or with unknown EBV serostatus.
|
Overview
Belatacept is a {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Adult Kidney Transplant Recipients
- NULOJIX® (belatacept) is indicated for prophylaxis of organ rejection in adult patients receiving a kidney transplant. NULOJIX is to be used in combination with basiliximab induction, mycophenolate mofetil, and corticosteroids.
Limitations of Use
- Use NULOJIX only in patients who are EBV seropositive.
- Use of NULOJIX for the prophylaxis of organ rejection in transplanted organs other than kidney has not been established.
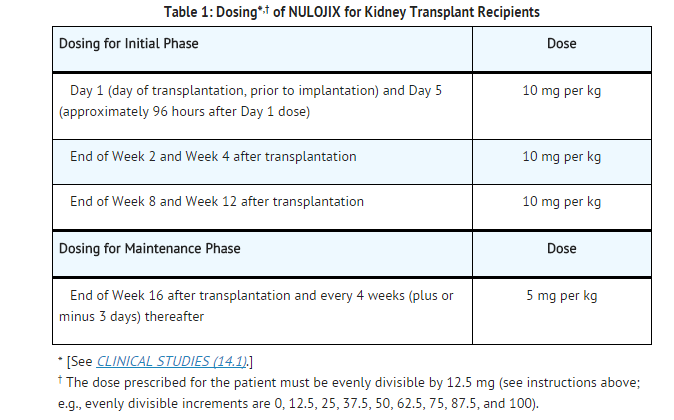
Dosage
Dosage in Adult Kidney Transplant Recipients
- NULOJIX should be administered in combination with basiliximab induction, mycophenolate mofetil (MMF), and corticosteroids. In clinical trials the median (25th-75th percentile) corticosteroid doses were tapered to approximately 15 mg (10-20 mg) per day by the first 6 weeks and remained at approximately 10 mg (5-10 mg) per day for the first 6 months post-transplant. Corticosteroid utilization should be consistent with the NULOJIX clinical trial experience.
- Due to an increased risk of post-transplant lymphoproliferative disorder (PTLD) predominantly involving the central nervous system (CNS), progressive multifocal leukoencephalopathy (PML), and serious CNS infections, administration of higher than the recommended doses or more frequent dosing of NULOJIX is not recommended.
- NULOJIX is for intravenous infusion only. Patients do not require premedication prior to administration of NULOJIX.
- Dosing instructions are provided in Table 1.
- The total infusion dose of NULOJIX should be based on the actual body weight of the patient at the time of transplantation, and should not be modified during the course of therapy, unless there is a change in body weight of greater than 10%.
- The prescribed dose of NULOJIX must be evenly divisible by 12.5 mg in order for the dose to be prepared accurately using the reconstituted solution and the silicone-free disposable syringe provided. Evenly divisible increments are 0, 12.5, 25, 37.5, 50, 62.5, 75, 87.5, and 100. For example:
- A patient weighs 64 kg. The dose is 10 mg per kg.
- Calculated Dose: 64 kg × 10 mg per kg = 640 mg
- The closest doses evenly divisible by 12.5 mg below and above 640 mg are 637.5 mg and 650 mg.
- The nearest dose to 640 mg is 637.5 mg.
- Therefore, the actual prescribed dose for the patient should be 637.5 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Belatacept in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Belatacept in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Belatacept in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Belatacept in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Belatacept in pediatric patients.
Contraindications
There is limited information regarding Belatacept Contraindications in the drug label.
Warnings
|
WARNING: POST-TRANSPLANT LYMPHOPROLIFERATIVE DISORDER, OTHER MALIGNANCIES, AND SERIOUS INFECTIONS
See full prescribing information for complete Boxed Warning.
* Increased risk for developing post-transplant lymphoproliferative disorder (PTLD), predominantly involving the central nervous system (CNS). Recipients without immunity to Epstein-Barr virus (EBV) are at a particularly increased risk; therefore, use in EBV seropositive patients only. Do not use NULOJIX in transplant recipients who are EBV seronegative or with unknown EBV serostatus.
|
There is limited information regarding Belatacept Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Belatacept in the drug label.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Belatacept in the drug label.
Drug Interactions
There is limited information regarding Belatacept Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Belatacept in women who are pregnant.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Belatacept in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Belatacept during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Belatacept with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Belatacept with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Belatacept with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Belatacept with respect to specific gender populations.
Race
There is no FDA guidance on the use of Belatacept with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Belatacept in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Belatacept in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Belatacept in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Belatacept in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Preparation and Administration Instructions
- NULOJIX is for intravenous infusion only.
- Caution: NULOJIX must be reconstituted/prepared using only the silicone-free disposable syringe provided with each vial.
- If the silicone-free disposable syringe is dropped or becomes contaminated, use a new silicone-free disposable syringe from inventory. For information on obtaining additional silicone-free disposable syringes, contact Bristol-Myers Squibb at 1-888-NULOJIX.
Preparation for Administration
- Calculate the number of NULOJIX vials required to provide the total infusion dose. Each vial contains 250 mg of belatacept lyophilized powder.
- Reconstitute the contents of each vial of NULOJIX with 10.5 mL of a suitable diluent using the silicone-free disposable syringe provided with each vial and an 18- to 21-gauge needle. Suitable diluents include: sterile water for injection (SWFI), 0.9% sodium chloride (NS), or 5% dextrose in water (D5W).
Note: If the NULOJIX powder is accidentally reconstituted using a different syringe than the one provided, the solution may develop a few translucent particles. Discard any solutions prepared using siliconized syringes.
- To reconstitute the NULOJIX powder, remove the flip-top from the vial and wipe the top with an alcohol swab. Insert the syringe needle into the vial through the center of the rubber stopper and direct the stream of diluent (10.5 mL of SWFI, NS, or D5W) to the glass wall of the vial.
- To minimize foam formation, rotate the vial and invert with gentle swirling until the contents are completely dissolved. Avoid prolonged or vigorous agitation. Do not shake.
- The reconstituted solution contains a belatacept concentration of 25 mg/mL and should be clear to slightly opalescent and colorless to pale yellow. Do not use if opaque particles, discoloration, or other foreign particles are present.
- Calculate the total volume of the reconstituted 25 mg/mL NULOJIX solution required to provide the total infusion dose.
Volume of 25 mg/mL NULOJIX solution (in mL) = Prescribed Dose (in mg) ÷ 25 mg/mL
- Prior to intravenous infusion, the required volume of the reconstituted NULOJIX solution must be further diluted with a suitable infusion fluid (NS or D5W). NULOJIX reconstituted with:
- SWFI should be further diluted with either NS or D5W
- NS should be further diluted with NS
- D5W should be further diluted with D5W
- From the appropriate size infusion bag or bottle, withdraw a volume of infusion fluid that is equal to the volume of the reconstituted NULOJIX solution required to provide the prescribed dose. With the same silicone-free disposable syringe used for reconstitution, withdraw the required amount of belatacept solution from the vial, inject it into the infusion bag or bottle, and gently rotate the infusion bag or bottle to ensure mixing.
The final belatacept concentration in the infusion bag or bottle should range from 2 mg/mL to 10 mg/mL. Typically, an infusion volume of 100 mL will be appropriate for most patients and doses, but total infusion volumes ranging from 50 mL to 250 mL may be used. Any unused solution remaining in the vials must be discarded.
- Prior to administration, the NULOJIX infusion should be inspected visually for particulate matter and discoloration. Discard the infusion if any particulate matter or discoloration is observed.
- The entire NULOJIX infusion should be administered over a period of 30 minutes and must be administered with an infusion set and a sterile, non-pyrogenic, low-protein-binding filter (with a pore size of 0.2-1.2 µm).
- The reconstituted solution should be transferred from the vial to the infusion bag or bottle immediately. The NULOJIX infusion must be completed within 24 hours of reconstitution of the NULOJIX lyophilized powder. If not used immediately, the infusion solution may be stored under refrigeration conditions: 2°-8°C (36°-46°F) and protected from light for up to 24 hours (a maximum of 4 hours of the total 24 hours can be at room temperature: 20°-25°C [68°-77°F] and room light).
- Infuse NULOJIX in a separate line from other concomitantly infused agents. NULOJIX should not be infused concomitantly in the same intravenous line with other agents. No physical or biochemical compatibility studies have been conducted to evaluate the coadministration of NULOJIX with other agents.
Monitoring
There is limited information regarding Monitoring of Belatacept in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Belatacept in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Belatacept in the drug label.
Pharmacology
There is limited information regarding Belatacept Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Belatacept Mechanism of Action in the drug label.
Structure
There is limited information regarding Belatacept Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Belatacept in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Belatacept in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Belatacept in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Belatacept in the drug label.
How Supplied
Storage
There is limited information regarding Belatacept Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Belatacept |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Belatacept |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Belatacept in the drug label.
Precautions with Alcohol
- Alcohol-Belatacept interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- NULOJIX
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Belatacept
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Belatacept |Label Name=Belatacept11.png
}}
{{#subobject:
|Label Page=Belatacept |Label Name=Belatacept11.png
}}