BRENZAVVY- bexagliflozin: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag=Kosar Doraghi, M.D. [mailto:k.doraghi@yahoo.com] |genericName=BRENZAVVY- bexagliflozin |aOrAn=a |drugClass=sodium-glucose co-transporter 2 (SGLT2) inhibitor |indicationType=treatment |indication=glycemic control in adults with type 2 diabetes mellitus |hasBlackBoxWarning=Yes |adverseReactions=female genital mycotic infections, urinary tract infection and increased urination |blackBoxWarningTitle=Warnings |blackBoxWarningBody=*Not re...") |
No edit summary |
||
| Line 3: | Line 3: | ||
|genericName=BRENZAVVY- bexagliflozin | |genericName=BRENZAVVY- bexagliflozin | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=sodium-glucose co-transporter 2 (SGLT2) inhibitor | |drugClass=[[sodium-glucose co-transporter 2 (SGLT2) inhibitor]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=glycemic control in adults with type 2 diabetes mellitus | |indication=glycemic control in adults with [[type 2 diabetes mellitus]] | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=female genital | |adverseReactions=female [[genital fungal infections]], [[urinary tract infection]] and increased urination | ||
|blackBoxWarningTitle=Warnings | |blackBoxWarningTitle=Warnings | ||
|blackBoxWarningBody=*Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. | |blackBoxWarningBody=*Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus. | ||
*Lower limb amputation: Consider factors that may increase the risk for amputations before initiating BRENZAVVY. Monitor patients for signs and symptoms of infection, or ulcers of the lower limbs, and discontinue if these occur | *Lower limb amputation: Consider factors that may increase the risk for amputations before initiating BRENZAVVY. Monitor patients for signs and symptoms of infection, or ulcers of the lower limbs, and discontinue if these occur | ||
*Volume depletion | *Volume depletion | ||
*Urosepsis and pyelonephritis | *Urosepsis and [[pyelonephritis]] | ||
*Hypoglycemia | *[[Hypoglycemia]] | ||
*Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) | *[[Necrotizing Fasciitis]] of the Perineum (Fournier’s Gangrene) | ||
*Genital mycotic infection | *Genital mycotic infection | ||
|fdaLIADAdult=20 mg once daily, taken in the morning, with or without food. Do not crush or chew the tablet. | |fdaLIADAdult=20 mg once daily, taken in the morning, with or without food. Do not crush or chew the tablet. | ||
| Line 21: | Line 21: | ||
*Withhold BRENZAVVY for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting | *Withhold BRENZAVVY for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting | ||
|contraindications=Hypersensitivity to bexagliflozin or any excipient in BRENZAVVY | |contraindications=Hypersensitivity to bexagliflozin or any excipient in BRENZAVVY | ||
|warnings=*Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis: Consider ketone monitoring in patients at risk for ketoacidosis. Assess for ketoacidosis regardless of presenting blood glucose levels and discontinue BRENZAVVY if ketoacidosis is suspected. Monitor patients for resolution of ketoacidosis before restarting. | |warnings=*[[Diabetic Ketoacidosis]] in Patients with Type 1 Diabetes Mellitus and Other [[Ketoacidosis]]: Consider ketone monitoring in patients at risk for ketoacidosis. Assess for ketoacidosis regardless of presenting blood glucose levels and discontinue BRENZAVVY if ketoacidosis is suspected. Monitor patients for resolution of ketoacidosis before restarting. | ||
*Lower limb amputation: Consider factors that may increase the risk for amputations before initiating BRENZAVVY. Monitor patients for signs and symptoms of infection or ulcers of the lower limbs, and discontinue if these occur. | *Lower limb amputation: Consider factors that may increase the risk for amputations before initiating BRENZAVVY. Monitor patients for signs and symptoms of infection or ulcers of the lower limbs, and discontinue if these occur. | ||
*Volume depletion: May result in acute kidney injury. Before initiating BRENZAVVY, assess and correct volume status in patients with impaired renal function or low systolic blood pressure, elderly patients, or patients on diuretics. Monitor for signs and symptoms during therapy. | *Volume depletion: May result in [[acute kidney injury]]. Before initiating BRENZAVVY, assess and correct volume status in patients with impaired renal function or low systolic blood pressure, elderly patients, or patients on diuretics. Monitor for signs and symptoms during therapy. | ||
*Urosepsis and pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly if indicated. | *Urosepsis and pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly if indicated. | ||
*Hypoglycemia: Consider a lower dose of insulin or insulin secretagogue to reduce the risk of hypoglycemia when used in combination with BRENZAVVY. | *Hypoglycemia: Consider a lower dose of [[insulin]] or insulin secretagogue to reduce the risk of [[hypoglycemia]] when used in combination with BRENZAVVY. | ||
*Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene): Serious, life-threatening cases have occurred in both females and males treated with SGLT2 inhibitors. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment. | *Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene): Serious, life-threatening cases have occurred in both females and males treated with [[SGLT2 inhibitors]]. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with [[fever]] or [[malaise]]. If suspected, institute prompt treatment. | ||
*Genital mycotic infection: Monitor and treat as appropriate. | *Genital mycotic infection: Monitor and treat as appropriate. | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
Pool of Placebo-Controlled Trials Evaluating BRENZAVVY 20 mg | Pool of Placebo-Controlled Trials Evaluating BRENZAVVY 20 mg | ||
The data in TABLE 1 are derived from three trials in adults with type 2 diabetes mellitus: two 24-week placebo-controlled trials (one as monotherapy and another as add-on to metformin therapy; Trials 1 and 2, respectively) and a 12-week, placebo-controlled, dose-ranging, monotherapy trial (only the data from the 20 mg dosage of BRENZAVVY per day were included in this pool). In these pooled trials, patients received placebo (N = 300) or BRENZAVVY 20 mg (N = 372), once daily. The mean age of the population was 56 years and 5% of the patients were older than 75 years of age. Fifty-seven percent (57%) were male and 45% were White, 38% Asian, 15% Black, and 2% other races. At baseline, the mean duration of type 2 diabetes mellitus was 7.7 years and the mean hemoglobin A1c (HbA1c) was 8.2%. Established microvascular complications of type 2 diabetes mellitus at baseline included diabetic nephropathy (0.8%), retinopathy (24%), and peripheral neuropathy (33%). Baseline renal function was eGFR ≥ 60 mL/min/1.73 m2 in 98% of patients and eGFR 45 to < 60 mL/min/1.73 m2 in 2% of patients (mean eGFR 92 mL/min/1.73 m2). | The data in TABLE 1 are derived from three trials in adults with type 2 diabetes mellitus: two 24-week placebo-controlled trials (one as monotherapy and another as add-on to metformin therapy; Trials 1 and 2, respectively) and a 12-week, placebo-controlled, dose-ranging, monotherapy trial (only the data from the 20 mg dosage of BRENZAVVY per day were included in this pool). In these pooled trials, patients received placebo (N = 300) or BRENZAVVY 20 mg (N = 372), once daily. The mean age of the population was 56 years and 5% of the patients were older than 75 years of age. Fifty-seven percent (57%) were male and 45% were White, 38% Asian, 15% Black, and 2% other races. At baseline, the mean duration of type 2 diabetes mellitus was 7.7 years and the mean [[hemoglobin A1c]] (HbA1c) was 8.2%. Established microvascular complications of type 2 diabetes mellitus at baseline included diabetic nephropathy (0.8%), retinopathy (24%), and peripheral neuropathy (33%). Baseline renal function was eGFR ≥ 60 mL/min/1.73 m2 in 98% of patients and eGFR 45 to < 60 mL/min/1.73 m2 in 2% of patients (mean eGFR 92 mL/min/1.73 m2). | ||
*common adverse reactions associated with the use of BRENZAVVY in these trials. These adverse reactions occurred more commonly in BRENZAVVY-treated patients than placebo-treated patients, and occurred in at least 2% of BRENZAVVY-treated patients. | *common adverse reactions associated with the use of BRENZAVVY in these trials. These adverse reactions occurred more commonly in BRENZAVVY-treated patients than placebo-treated patients, and occurred in at least 2% of BRENZAVVY-treated patients. | ||
*Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis | *Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis | ||
| Line 40: | Line 40: | ||
*Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) | *Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene) | ||
*Genital Mycotic Infections | *Genital Mycotic Infections | ||
|postmarketing=*Changes in Serum Creatinine and eGFR: | |postmarketing=*Changes in Serum Creatinine and [[eGFR]]: | ||
Initiation of BRENZAVVY leads to an initial increase in serum creatinine and decrease in eGFR within weeks of starting therapy, stabilizing by week 6 to 12. In a trial involving adults with type 2 diabetes mellitus and moderate renal impairment, BRENZAVVY resulted in a mean increase in serum creatinine of 0.1 mg/dL and a decrease in eGFR of 4.6 mL/min/1.73 m2 within the first 6 weeks compared to placebo. In another trial with adults at risk for cardiovascular disease, BRENZAVVY showed an initial decrease in eGFR. These changes are reversed upon treatment discontinuation, indicating possible acute hemodynamic effects. | Initiation of BRENZAVVY leads to an initial increase in serum creatinine and decrease in eGFR within weeks of starting therapy, stabilizing by week 6 to 12. In a trial involving adults with type 2 diabetes mellitus and moderate renal impairment, BRENZAVVY resulted in a mean increase in serum creatinine of 0.1 mg/dL and a decrease in eGFR of 4.6 mL/min/1.73 m2 within the first 6 weeks compared to placebo. In another trial with adults at risk for cardiovascular disease, BRENZAVVY showed an initial decrease in eGFR. These changes are reversed upon treatment discontinuation, indicating possible acute hemodynamic effects. | ||
*Increases in Low-Density Lipoprotein Cholesterol (LDL-C): | *Increases in Low-Density [[Lipoprotein Cholesterol]] (LDL-C): | ||
In a pool of two placebo-controlled trials, BRENZAVVY treatment resulted in a slight increase in LDL-C compared to placebo at week 24. Similarly, in a trial with adults at risk for cardiovascular disease, LDL-C increased with BRENZAVVY treatment. | In a pool of two placebo-controlled trials, BRENZAVVY treatment resulted in a slight increase in LDL-C compared to placebo at week 24. Similarly, in a trial with adults at risk for cardiovascular disease, LDL-C increased with BRENZAVVY treatment. | ||
*Increases in Hemoglobin and Hematocrit: | *Increases in Hemoglobin and Hematocrit: | ||
| Line 78: | Line 78: | ||
|clinicalStudies=BRENZAVVY has been studied as monotherapy (Trial 1) and in combination with metformin in adults with type 2 diabetes mellitus (Trials 2, 3, and 4) BRENZAVVY has also been studied in adults with type 2 diabetes mellitus with moderate renal impairment (Trial 5) and in adults with type 2 diabetes mellitus with established CVD or at increased risk for CVD (Trial 6) | |clinicalStudies=BRENZAVVY has been studied as monotherapy (Trial 1) and in combination with metformin in adults with type 2 diabetes mellitus (Trials 2, 3, and 4) BRENZAVVY has also been studied in adults with type 2 diabetes mellitus with moderate renal impairment (Trial 5) and in adults with type 2 diabetes mellitus with established CVD or at increased risk for CVD (Trial 6) | ||
Treatment with BRENZAVVY reduced hemoglobin A1c (HbA1c) compared to placebo and efficacy was noninferior to glimepiride (up-titrated to a maximum dose of 6 mg) and sitagliptin 100 mg once daily (see Trials 3 and 4). The reduction in HbA1c by BRENZAVVY was shown across subgroups of age, sex, race, and geographic region. | Treatment with BRENZAVVY reduced hemoglobin A1c (HbA1c) compared to placebo and efficacy was noninferior to glimepiride (up-titrated to a maximum dose of 6 mg) and sitagliptin 100 mg once daily (see Trials 3 and 4). The reduction in HbA1c by BRENZAVVY was shown across subgroups of age, sex, race, and geographic region. | ||
A total of 207 adults with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) by diet and exercise participated in a randomized, double-blind, multi-center, 24-week, placebo-controlled trial (NCT02715258; referred to as Trial 1) to evaluate the efficacy of BRENZAVVY monotherapy. Patients were either treatment naïve or had discontinued a single oral antihyperglycemic treatment ≥ 6 weeks prior to entering a 2-week, single-blind, placebo run-in period. Upon completion of the run-in period they were randomized (1:2) to placebo or BRENZAVVY 20 mg administered orally once daily. The mean age of the population was 55 years and 4% of the patients were older than 75 years of age. Forty-eight percent (48%) were male and 74% were White, 10% were Asian, 15% were Black and 1% were other races. Fifty-two percent (52%) were Hispanic/Latino. | A total of 207 adults with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) by diet and exercise participated in a randomized, double-blind, multi-center, 24-week, placebo-controlled trial (NCT02715258; referred to as Trial 1) to evaluate the efficacy of BRENZAVVY monotherapy. Patients were either treatment naïve or had discontinued a single oral antihyperglycemic treatment ≥ 6 weeks prior to entering a 2-week, single-blind, placebo run-in period. Upon completion of the run-in period they were randomized (1:2) to placebo or BRENZAVVY 20 mg administered orally once daily. The mean age of the population was 55 years and 4% of the patients were older than 75 years of age. Forty-eight percent (48%) were male and 74% were White, 10% were Asian, 15% were Black and 1% were other races. Fifty-two percent (52%) were Hispanic/Latino. Same model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate. | ||
[[Image:IMG 1052.jpeg|400px]] | |||
Vertical lines represent 95% confidence intervals. Treatment group least squares mean changes from baseline in HbA1c were estimated by an ANCOVA model using observed data for intermediate visits. Numbers of patients per arm per measurement shown below plot. Return-to-baseline (RTB) analysis results at Week 24 are plotted to the right separately. | Vertical lines represent 95% confidence intervals. Treatment group least squares mean changes from baseline in HbA1c were estimated by an ANCOVA model using observed data for intermediate visits. Numbers of patients per arm per measurement shown below plot. Return-to-baseline (RTB) analysis results at Week 24 are plotted to the right separately. | ||
|howSupplied=BRENZAVVY 20 mg tablets are blue, caplet-shaped, biconvex, bevel-edged, film | |howSupplied=BRENZAVVY 20 mg tablets are blue, caplet-shaped, biconvex, bevel-edged, film | ||
Latest revision as of 04:03, 21 April 2024
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kosar Doraghi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warnings
See full prescribing information for complete Boxed Warning.
*Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus.
|
Overview
BRENZAVVY- bexagliflozin is a sodium-glucose co-transporter 2 (SGLT2) inhibitor that is FDA approved for the treatment of glycemic control in adults with type 2 diabetes mellitus. There is a Black Box Warning for this drug as shown here. Common adverse reactions include female genital fungal infections, urinary tract infection and increased urination.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
20 mg once daily, taken in the morning, with or without food. Do not crush or chew the tablet. Assess renal function before initiating BRENZAVVY and as clinically indicated. Correct volume depletion before initiating
- Not recommended if eGFR less than 30 mL/min/1.73 m2. (2.1)
- Withhold BRENZAVVY for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding BRENZAVVY- bexagliflozin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
Hypersensitivity to bexagliflozin or any excipient in BRENZAVVY
Warnings
|
Warnings
See full prescribing information for complete Boxed Warning.
*Not recommended for use to improve glycemic control in patients with type 1 diabetes mellitus.
|
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis: Consider ketone monitoring in patients at risk for ketoacidosis. Assess for ketoacidosis regardless of presenting blood glucose levels and discontinue BRENZAVVY if ketoacidosis is suspected. Monitor patients for resolution of ketoacidosis before restarting.
- Lower limb amputation: Consider factors that may increase the risk for amputations before initiating BRENZAVVY. Monitor patients for signs and symptoms of infection or ulcers of the lower limbs, and discontinue if these occur.
- Volume depletion: May result in acute kidney injury. Before initiating BRENZAVVY, assess and correct volume status in patients with impaired renal function or low systolic blood pressure, elderly patients, or patients on diuretics. Monitor for signs and symptoms during therapy.
- Urosepsis and pyelonephritis: Evaluate patients for signs and symptoms of urinary tract infections and treat promptly if indicated.
- Hypoglycemia: Consider a lower dose of insulin or insulin secretagogue to reduce the risk of hypoglycemia when used in combination with BRENZAVVY.
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene): Serious, life-threatening cases have occurred in both females and males treated with SGLT2 inhibitors. Assess patients presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise. If suspected, institute prompt treatment.
- Genital mycotic infection: Monitor and treat as appropriate.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials Evaluating BRENZAVVY 20 mg The data in TABLE 1 are derived from three trials in adults with type 2 diabetes mellitus: two 24-week placebo-controlled trials (one as monotherapy and another as add-on to metformin therapy; Trials 1 and 2, respectively) and a 12-week, placebo-controlled, dose-ranging, monotherapy trial (only the data from the 20 mg dosage of BRENZAVVY per day were included in this pool). In these pooled trials, patients received placebo (N = 300) or BRENZAVVY 20 mg (N = 372), once daily. The mean age of the population was 56 years and 5% of the patients were older than 75 years of age. Fifty-seven percent (57%) were male and 45% were White, 38% Asian, 15% Black, and 2% other races. At baseline, the mean duration of type 2 diabetes mellitus was 7.7 years and the mean hemoglobin A1c (HbA1c) was 8.2%. Established microvascular complications of type 2 diabetes mellitus at baseline included diabetic nephropathy (0.8%), retinopathy (24%), and peripheral neuropathy (33%). Baseline renal function was eGFR ≥ 60 mL/min/1.73 m2 in 98% of patients and eGFR 45 to < 60 mL/min/1.73 m2 in 2% of patients (mean eGFR 92 mL/min/1.73 m2).
- common adverse reactions associated with the use of BRENZAVVY in these trials. These adverse reactions occurred more commonly in BRENZAVVY-treated patients than placebo-treated patients, and occurred in at least 2% of BRENZAVVY-treated patients.
- Diabetic Ketoacidosis in Patients with Type 1 Diabetes Mellitus and Other Ketoacidosis
- Lower Limb Amputation
- Volume Depletion
- Urosepsis and Pyelonephritis
- Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
- Necrotizing Fasciitis of the Perineum (Fournier’s Gangrene)
- Genital Mycotic Infections
Postmarketing Experience
- Changes in Serum Creatinine and eGFR:
Initiation of BRENZAVVY leads to an initial increase in serum creatinine and decrease in eGFR within weeks of starting therapy, stabilizing by week 6 to 12. In a trial involving adults with type 2 diabetes mellitus and moderate renal impairment, BRENZAVVY resulted in a mean increase in serum creatinine of 0.1 mg/dL and a decrease in eGFR of 4.6 mL/min/1.73 m2 within the first 6 weeks compared to placebo. In another trial with adults at risk for cardiovascular disease, BRENZAVVY showed an initial decrease in eGFR. These changes are reversed upon treatment discontinuation, indicating possible acute hemodynamic effects.
- Increases in Low-Density Lipoprotein Cholesterol (LDL-C):
In a pool of two placebo-controlled trials, BRENZAVVY treatment resulted in a slight increase in LDL-C compared to placebo at week 24. Similarly, in a trial with adults at risk for cardiovascular disease, LDL-C increased with BRENZAVVY treatment.
- Increases in Hemoglobin and Hematocrit:
BRENZAVVY treatment led to increases in hemoglobin and hematocrit levels compared to placebo in trials. Fewer patients experienced significant increases in hemoglobin with placebo compared to BRENZAVVY, indicating a potential effect on erythropoiesis.
Drug Interactions
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
BRENZAVVY is not recommended during the second and third trimesters of pregnancy
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of BRENZAVVY- bexagliflozin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of BRENZAVVY- bexagliflozin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of BRENZAVVY- bexagliflozin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of BRENZAVVY- bexagliflozin in pediatric settings.
Geriatic Use
Higher incidence of adverse reactions related to volume depletion
Gender
There is no FDA guidance on the use of BRENZAVVY- bexagliflozin with respect to specific gender populations.
Race
There is no FDA guidance on the use of BRENZAVVY- bexagliflozin with respect to specific racial populations.
Renal Impairment
Higher incidence of adverse reactions related to reduced renal function
Hepatic Impairment
Not recommended for patients with severe hepatic impairmen
Females of Reproductive Potential and Males
There is no FDA guidance on the use of BRENZAVVY- bexagliflozin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of BRENZAVVY- bexagliflozin in patients who are immunocompromised.
Administration and Monitoring
Administration
Testing Prior to Initiation and During Treatment with BRENZAVVY
- Assess renal function prior to initiation of BRENZAVVY and periodically thereafter as clinically indicated. BRENZAVVY is not recommended in patients with an eGFR less than 30 mL/min/1.73 m2.
- Assess volume status. In patients with volume depletion, correct this condition before initiating BRENZAVVY.
Recommended Dosage
- The recommended dosage of BRENZAVVY is 20 mg orally taken once daily in the morning, with or without food.
- Do not crush or chew the tablet.
- If a dose is missed, take the missed dose as soon as possible. Do not double the next dose.
- Temporary Interruption for Surgery
Withhold BRENZAVVY for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting. Resume BRENZAVVY when the patient is clinically stable and has resumed oral intake.
Monitoring
There is limited information regarding BRENZAVVY- bexagliflozin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of BRENZAVVY- bexagliflozin and IV administrations.
Overdosage
There is limited information regarding BRENZAVVY- bexagliflozin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding BRENZAVVY- bexagliflozin Pharmacology in the drug label.
Mechanism of Action
Bexagliflozin is an inhibitor of sodium-glucose co-transporter 2 (SGLT2), the transporter responsible for reabsorption of the majority of glucose from the renal glomerular filtrate in the renal proximal tubule. By inhibiting SGLT2, bexagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Structure
BRENZAVVY tablets for oral use contain bexagliflozin, an SGLT2 inhibitor.
The chemical name of bexagliflozin is (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol.
The molecular formula is C24H29ClO7 and the molecular weight is 464.94 g/mol. The structural formula is: 
Pharmacodynamics
Urinary Glucose Excretion and Urinary Volume Dose-dependent increases in urinary glucose excretion (UGE) accompanied by increases in urine volume were observed in healthy subjects and in adults with type 2 diabetes mellitus following single- and multiple-dose administration of bexagliflozin. Dose-response analysis indicates that 20 mg bexagliflozin provides near-maximal UGE. Elevated UGE was maintained after multiple-dose administration. Cardiac Electrophysiology At 5 times the recommended dose, bexagliflozin does not prolong the QTc interval to any clinically significant extent.
Pharmacokinetics
The pharmacokinetics of bexagliflozin are similar in healthy subjects and adults with type 2 diabetes mellitus. Following dosing in the fasted state, mean Cmax and AUC0-∞ were 134 ng/mL and 1,162 ng·h/mL, respectively. Bexagliflozin does not exhibit time-dependent pharmacokinetics and accumulates in plasma up to ~20% following multiple dosing.
- Absorption
Following oral administration of BRENZAVVY, peak plasma concentrations of bexagliflozin were reached between 2 – 4 hours post-dose and can be delayed if taken after a meal or by medications that slow gastric emptying Elimination
Metabolism Bexagliflozin is mainly metabolized by UGT1A9 and, to a lesser extent, CYP3A. In plasma the most abundant metabolite is the pharmacologically inactive 3′-O-glucuronide, which was found to constitute 32.2% of the parent compound AUC in a radiolabeled tracer study. None of the metabolites are expected to have clinically relevant pharmacological effects.
Nonclinical Toxicology
There is limited information regarding BRENZAVVY- bexagliflozin Nonclinical Toxicology in the drug label.
Clinical Studies
BRENZAVVY has been studied as monotherapy (Trial 1) and in combination with metformin in adults with type 2 diabetes mellitus (Trials 2, 3, and 4) BRENZAVVY has also been studied in adults with type 2 diabetes mellitus with moderate renal impairment (Trial 5) and in adults with type 2 diabetes mellitus with established CVD or at increased risk for CVD (Trial 6)
Treatment with BRENZAVVY reduced hemoglobin A1c (HbA1c) compared to placebo and efficacy was noninferior to glimepiride (up-titrated to a maximum dose of 6 mg) and sitagliptin 100 mg once daily (see Trials 3 and 4). The reduction in HbA1c by BRENZAVVY was shown across subgroups of age, sex, race, and geographic region.
A total of 207 adults with type 2 diabetes mellitus inadequately controlled (HbA1c between 7% and 10.5%) by diet and exercise participated in a randomized, double-blind, multi-center, 24-week, placebo-controlled trial (NCT02715258; referred to as Trial 1) to evaluate the efficacy of BRENZAVVY monotherapy. Patients were either treatment naïve or had discontinued a single oral antihyperglycemic treatment ≥ 6 weeks prior to entering a 2-week, single-blind, placebo run-in period. Upon completion of the run-in period they were randomized (1:2) to placebo or BRENZAVVY 20 mg administered orally once daily. The mean age of the population was 55 years and 4% of the patients were older than 75 years of age. Forty-eight percent (48%) were male and 74% were White, 10% were Asian, 15% were Black and 1% were other races. Fifty-two percent (52%) were Hispanic/Latino. Same model as for HbA1c endpoint but with baseline FPG instead of baseline HbA1c as a covariate.
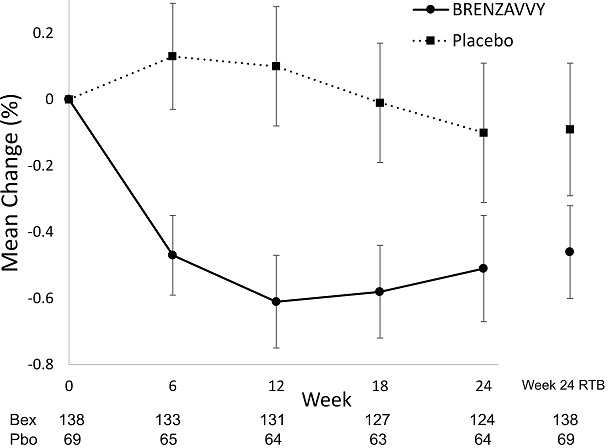 Vertical lines represent 95% confidence intervals. Treatment group least squares mean changes from baseline in HbA1c were estimated by an ANCOVA model using observed data for intermediate visits. Numbers of patients per arm per measurement shown below plot. Return-to-baseline (RTB) analysis results at Week 24 are plotted to the right separately.
Vertical lines represent 95% confidence intervals. Treatment group least squares mean changes from baseline in HbA1c were estimated by an ANCOVA model using observed data for intermediate visits. Numbers of patients per arm per measurement shown below plot. Return-to-baseline (RTB) analysis results at Week 24 are plotted to the right separately.
How Supplied
BRENZAVVY 20 mg tablets are blue, caplet-shaped, biconvex, bevel-edged, film

Storage
Store from 20 °C to 25 °C (68 °F to 77 °F); excursions permitted between 15 °C to 30 °C (59 °F to 86 °F)
Images
Drug Images
{{#ask: Page Name::BRENZAVVY- bexagliflozin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::BRENZAVVY- bexagliflozin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding BRENZAVVY- bexagliflozin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-BRENZAVVY- bexagliflozin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding BRENZAVVY- bexagliflozin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding BRENZAVVY- bexagliflozin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
