Azithromycin (ophthalmic): Difference between revisions
Kiran Singh (talk | contribs) No edit summary |
m (Protected "Azithromycin (ophthalmic)": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (7 intermediate revisions by one other user not shown) | |||
| Line 2: | Line 2: | ||
|authorTag={{KS}} | |authorTag={{KS}} | ||
|genericName=azithromycin monohydrate | |genericName=azithromycin monohydrate | ||
|aOrAn= | |aOrAn=an | ||
|drugClass=antibiotic | |||
|indicationType=treatment | |indicationType=treatment | ||
|adverseReactions= | |indication=[[bacterial]] [[conjunctivitis]] | ||
|adverseReactions=eye irritation and abnormal vision | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 31: | Line 33: | ||
* 2.5 mL of a 1% sterile topical ophthalmic solution. | * 2.5 mL of a 1% sterile topical ophthalmic solution. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 38: | Line 38: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=* The safety and effectiveness of AzaSite solution in pediatric patients below 1 year of age have not been established. The efficacy of AzaSite in treating bacterial conjunctivitis in pediatric patients one year or older has been demonstrated in controlled clinical trials. | ||
==Indications== | |||
* AzaSite® is indicated for the treatment of bacterial [[conjunctivitis]] caused by susceptible isolates of the following microorganisms: | |||
:*CDC coryneform group G1 | |||
:*[[Haemophilus influenzae]] | |||
:*[[Staphylococcus aureus]] | |||
:*Streptococcus mitis group | |||
:*[[Streptococcus pneumoniae]] | |||
==Dosage== | |||
* Instill 1 drop in the affected eye(s) twice daily, eight to twelve hours apart for the first two days and then instill 1 drop in the affected eye(s) once daily for the next five days. | |||
==DOSAGE FORMS AND STRENGTHS== | |||
* 2.5 mL of a 1% sterile topical ophthalmic solution. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|contraindications=* [[Hypersensitivity]] to any component of this product. | |contraindications=* [[Hypersensitivity]] to any component of this product. | ||
|warnings='''Topical Ophthalmic Use Only''' | |warnings='''Topical Ophthalmic Use Only''' | ||
| Line 66: | Line 77: | ||
|clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with the rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice. | |clinicalTrials=* Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with the rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice. | ||
* The data described below reflect exposure to AzaSite in 698 patients. The population was between 1 and 87 years old with clinical signs and symptoms of bacterial conjunctivitis. The most frequently reported ocular adverse reaction reported in patients receiving AzaSite was eye irritation. This reaction occurred in approximately 1-2% of patients. Other adverse reactions associated with the use of AzaSite were reported in less than 1% of patients and included ocular reactions ([[blurred vision]], [[burning]], stinging and irritation upon instillation, [[contact dermatitis]], corneal erosion, [[dry eye]], [[eye pain]], [[itching]], ocular discharge, punctate [[keratitis]], [[visual acuity]] reduction) and non-ocular reactions (dysgeusia, facial swelling, hives, nasal congestion, periocular swelling, rash, sinusitis, urticaria). | * The data described below reflect exposure to AzaSite in 698 patients. The population was between 1 and 87 years old with clinical signs and symptoms of bacterial conjunctivitis. The most frequently reported ocular adverse reaction reported in patients receiving AzaSite was eye irritation. This reaction occurred in approximately 1-2% of patients. Other adverse reactions associated with the use of AzaSite were reported in less than 1% of patients and included ocular reactions ([[blurred vision]], [[burning]], stinging and irritation upon instillation, [[contact dermatitis]], corneal erosion, [[dry eye]], [[eye pain]], [[itching]], ocular discharge, punctate [[keratitis]], [[visual acuity]] reduction) and non-ocular reactions (dysgeusia, facial swelling, hives, nasal [[congestion]], periocular swelling, [[rash]], [[sinusitis]], [[urticaria]]). | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | ||
|useInPregnancyFDA=* '''Pregnancy Category B'''. Reproduction studies have been performed in rats and mice at doses up to 200 mg/kg/day. The highest dose was associated with moderate maternal toxicity. These doses are estimated to be approximately 5,000 times the maximum human ocular daily dose of 2 mg. In the animal studies, no evidence of harm to the fetus due to azithromycin was found. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, azithromycin should be used during pregnancy only if clearly needed. | |||
|useInPregnancyFDA=* '''Pregnancy Category''' | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* It is not known whether azithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when azithromycin is administered to a nursing woman. | ||
|useInPed= | |useInPed=* The safety and effectiveness of AzaSite solution in pediatric patients below 1 year of age have not been established. The efficacy of AzaSite in treating bacterial conjunctivitis in pediatric patients one year or older has been demonstrated in controlled clinical trials | ||
|useInGeri= | |useInGeri=* No overall differences in safety or effectiveness have been observed between elderly and younger patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
| Line 140: | Line 93: | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
|administration=* Ophthalmic | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
|overdose=There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
|drugBox={{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 458267362 | |||
| IUPAC_name = (2''R'',3''S'',4''R'',5''R'',8''R'',10''R'',11''R'',12''S'',13''S'',14''R''<nowiki>)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-15-oxo- 11-{[3,4,6-trideoxy-3-(dimethylamino)-β-</nowiki><small>D</small>-''xylo''<nowiki>-hexopyranosyl]oxy}-1-oxa-6-azacyclopentadec-13-yl 2,6-dideoxy-3-</nowiki>''C''-methyl-3-''O''-methyl-α-<small>L</small>-''ribo''-hexopyranoside | |||
| image =Azithromycin structure.png | |||
| image2 =Azithromycin_3d_structure.png | |||
<!-- | <!--Clinical data--> | ||
| | | tradename = Zithromax, Azyth, Azithrocin, Azin, Zeto | ||
| Drugs.com = {{drugs.com|monograph|azithromycin}} | |||
| MedlinePlus = a697037 | |||
| licence_US = Azithromycin | |||
| pregnancy_AU = B1 | |||
| pregnancy_US = B | |||
| legal_US = Rx-only | |||
| routes_of_administration = [[Wiktionary:oral|Oral]] (capsule, tablet or suspension), [[intravenous therapy|intravenous]], [[eye drop|ophthalmic]] | |||
==== | <!--Pharmacokinetic data--> | ||
| bioavailability = 38% for 250 mg capsules | |||
| metabolism = [[Hepatic]] | |||
| elimination_half-life = 11–14 h (single dose) | |||
68 h (multiple dosing) | |||
| excretion = [[Biliary]], [[renal]] (4.5%) | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 83905-01-5 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = FA10 | |||
| ATC_supplemental = {{ATC|S01|AA26}} | |||
| PubChem = 55185 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00207 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 10482163 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = J2KLZ20U1M | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D07486 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 2955 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 529 | |||
| NIAID_ChemDB = 007311 | |||
==== | <!--Chemical data--> | ||
| C=38 | H=72 | N=2 | O=12 | |||
| molecular_weight = 748.984 g·mol<sup>−1</sup> | |||
| smiles = CN(C)[C@H]3C[C@@H](C)O[C@@H](O[C@@H]2[C@@H](C)[C@H](O[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1)[C@@H](C)C(=O)O[C@H](CC)[C@@](C)(O)[C@H](O)[C@@H](C)N(C)C[C@H](C)C[C@@]2(C)O)[C@@H]3O | |||
| InChI = 1/C38H72N2O12/c1-15-27-38(10,46)31(42)24(6)40(13)19-20(2)17-36(8,45)33(52-35-29(41)26(39(11)12)16-21(3)48-35)22(4)30(23(5)34(44)50-27)51-28-18-37(9,47-14)32(43)25(7)49-28/h20-33,35,41-43,45-46H,15-19H2,1-14H3/t20-,21-,22+,23-,24-,25+,26+,27-,28+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1 | |||
| InChIKey = MQTOSJVFKKJCRP-BICOPXKEBK | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C38H72N2O12/c1-15-27-38(10,46)31(42)24(6)40(13)19-20(2)17-36(8,45)33(52-35-29(41)26(39(11)12)16-21(3)48-35)22(4)30(23(5)34(44)50-27)51-28-18-37(9,47-14)32(43)25(7)49-28/h20-33,35,41-43,45-46H,15-19H2,1-14H3/t20-,21-,22+,23-,24-,25+,26+,27-,28+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = MQTOSJVFKKJCRP-BICOPXKESA-N | |||
| synonyms = <small>9-deoxy-9a-aza-9a-methyl-9a-homoerythromycin A</small> | |||
}} | |||
|mechAction=* Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and interfering with microbial protein synthesis. | |||
|structure=* AzaSite (azithromycin ophthalmic solution) is a 1% sterile aqueous topical ophthalmic solution of azithromycin formulated in DuraSite® (polycarbophil, edetate disodium, sodium chloride). AzaSite is an off-white, viscous liquid with an osmolality of approximately 290 mOsm/kg. | |||
* | * '''Preservative''': 0.003% benzalkonium chloride. Inactives: mannitol, citric acid, sodium citrate, poloxamer 407, polycarbophil, edetate disodium (EDTA), sodium chloride, water for injection, and sodium hydroxide to adjust pH to 6.3. | ||
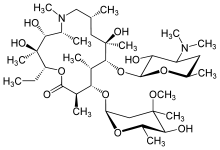
* Azithromycin is a macrolide antibiotic with a 15-membered ring. Its chemical name is (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyloxy]]-1-oxa-6-aza-cyclopentadecan-15-one, and the structural formula is: | |||
[[File:Azithromycin ophthalmic structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
< | * Azithromycin has a molecular weight of 749, and its empirical formula is C38H72N2O12. | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
< | |PK=* The plasma concentration of azithromycin following ocular administration of AzaSite (azithromycin ophthalmic solution) in humans is unknown. Based on the proposed dose of one drop to each eye (total dose of 100 mcL or 1 mg) and exposure information from systemic administration, the systemic concentration of azithromycin following ocular administration is estimated to be below quantifiable limits (≤10 ng/mL) at steady-state in humans, assuming 100% systemic availability. | ||
|nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | |||
| | |||
| | |||
: | * Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in standard laboratory tests: mouse lymphoma assay, human lymphocyte clastogenic assay, and mouse bone marrow clastogenic assay. No evidence of impaired fertility due to azithromycin was found in mice or rats that received oral doses of up to 200 mg/kg/day. | ||
|clinicalStudies=* In a randomized, vehicle-controlled, double-blind, multicenter clinical study in which patients were dosed twice daily for the first two days, then once daily on days 3, 4, and 5, AzaSite solution was superior to vehicle on days 6-7 in patients who had a confirmed clinical diagnosis of bacterial conjunctivitis. Clinical resolution was achieved in 63% (82/130) of patients treated with AzaSite versus 50% (74/149) of patients treated with vehicle. The p-value for the comparison was 0.03 and the 95% confidence interval around the 13% (63%-50%) difference was 2% to 25%. The microbiological success rate for the eradication of the baseline pathogens was approximately 88% compared to 66% of patients treated with vehicle (p<0.001, confidence interval around the 22% difference was 13% to 31%). Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials. | |||
|howSupplied=* AzaSite is a sterile aqueous topical ophthalmic formulation of 1% azithromycin. | |||
* NDC 31357-040-25: 2.5 mL in 5 mL bottle containing a total of 25 mg of azithromycin in a white, round, low-density polyethylene (LDPE) bottle, with a clear LDPE dropper tip, and a tan colored high density polyethylene (HDPE) eyedropper cap. A white tamper evident over-cap is provided. | |||
* NDC 31357-040-03: 2.5 mL in 4 mL bottle containing a total of 25 mg of azithromycin in a white, round, low-density polyethylene (LDPE) bottle, with a clear LDPE dropper tip, and a tan colored high density polyethylene (HDPE) eyedropper cap. A white tamper evident over-cap is provided. | |||
| | |storage=* Store unopened bottle under refrigeration at 2°C to 8°C (36°F to 46°F). Once the bottle is opened, store at 2°C to 25°C (36°F to 77°F) for up to 14 days. Discard after the 14 days. | ||
|packLabel=[[File:Azithromycin ophthalmic pt insert.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
[[File:Azithromycin ophthalmic instruction.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
| | |||
[[File:Azithromycin ophthalmic image.jpg|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
| | |||
[[File:Azithromycin ophthalmic ingredients and appearance.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
| | |||
| | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* AZASITE ®<ref>{{Cite web | title =azithromycin monohydrate solution| url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5dc0f75a-1e14-469f-af4f-c668a32f2328 }}</ref> | |brandNames=* AZASITE ®<ref>{{Cite web | title =azithromycin monohydrate solution| url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5dc0f75a-1e14-469f-af4f-c668a32f2328 }}</ref> | ||
|lookAlike=* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |lookAlike=* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | ||
| Line 222: | Line 213: | ||
[[Category:Macrolide antibiotics]] | |||
[[Category:Antimalarial agents]] | |||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 17:52, 18 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
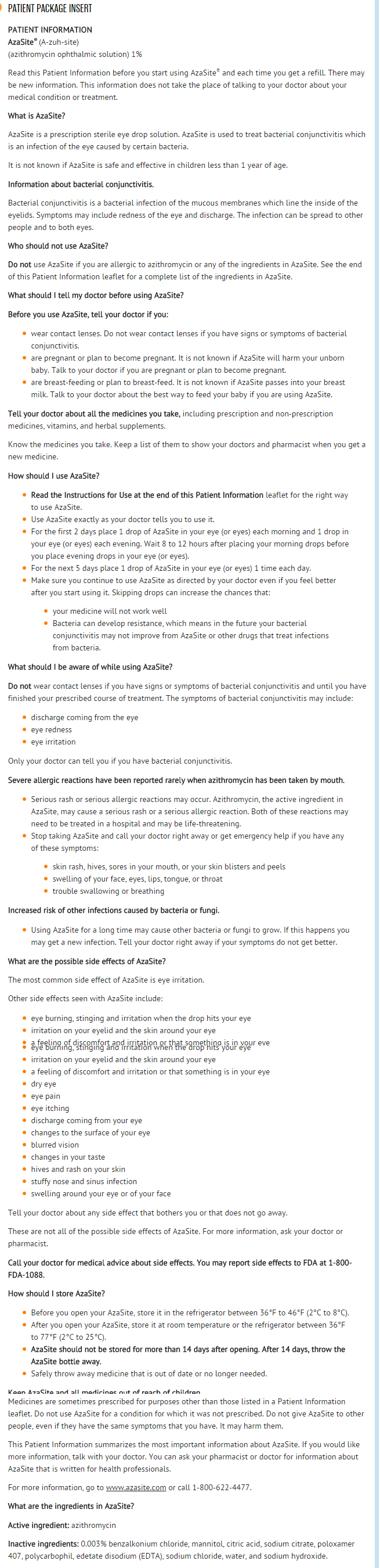
Azithromycin (ophthalmic) is an antibiotic that is FDA approved for the treatment of bacterial conjunctivitis. Common adverse reactions include eye irritation and abnormal vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- AzaSite® is indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following microorganisms:
- CDC coryneform group G1
- Haemophilus influenzae
- Staphylococcus aureus
- Streptococcus mitis group
- Streptococcus pneumoniae
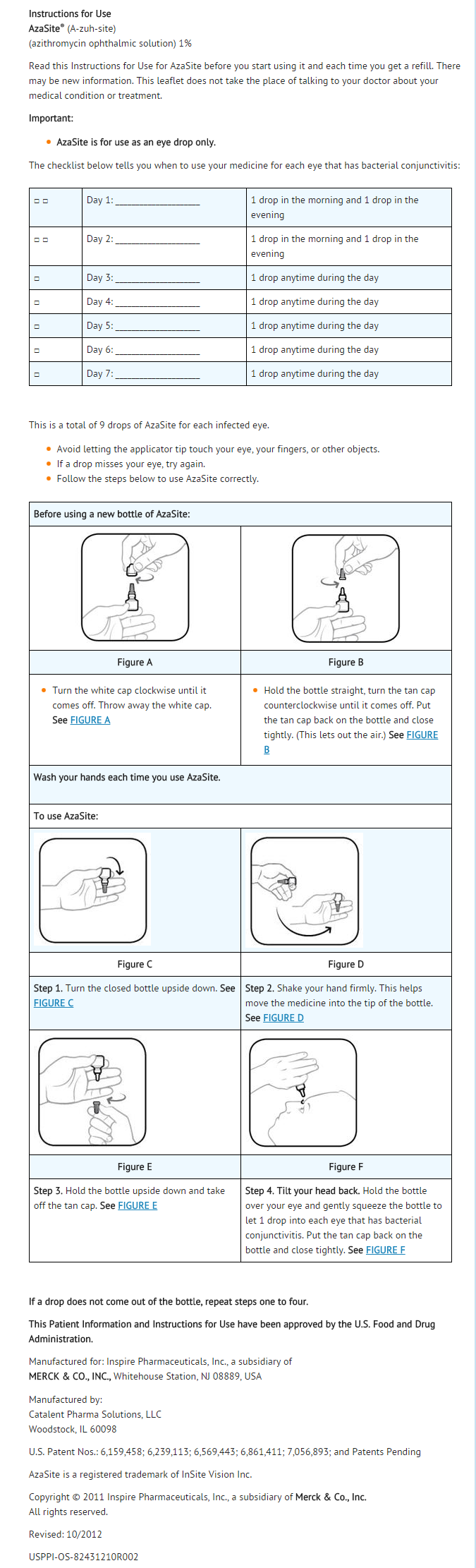
Dosage
- Instill 1 drop in the affected eye(s) twice daily, eight to twelve hours apart for the first two days and then instill 1 drop in the affected eye(s) once daily for the next five days.
DOSAGE FORMS AND STRENGTHS
- 2.5 mL of a 1% sterile topical ophthalmic solution.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Azithromycin (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Azithromycin (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- The safety and effectiveness of AzaSite solution in pediatric patients below 1 year of age have not been established. The efficacy of AzaSite in treating bacterial conjunctivitis in pediatric patients one year or older has been demonstrated in controlled clinical trials.
Indications
- AzaSite® is indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following microorganisms:
- CDC coryneform group G1
- Haemophilus influenzae
- Staphylococcus aureus
- Streptococcus mitis group
- Streptococcus pneumoniae
Dosage
- Instill 1 drop in the affected eye(s) twice daily, eight to twelve hours apart for the first two days and then instill 1 drop in the affected eye(s) once daily for the next five days.
DOSAGE FORMS AND STRENGTHS
- 2.5 mL of a 1% sterile topical ophthalmic solution.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Azithromycin (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Azithromycin (ophthalmic) in pediatric patients.
Contraindications
- Hypersensitivity to any component of this product.
Warnings
Topical Ophthalmic Use Only
- NOT FOR INJECTION. AzaSite is indicated for topical ophthalmic use only, and should not be administered systemically, injected subconjunctivally, or introduced directly into the anterior chamber of the eye.
Anaphylaxis and Hypersensitivity with Systemic Use of Azithromycin
- In patients receiving systemically administered azithromycin, serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported rarely in patients on azithromycin therapy. Although rare, fatalities have been reported. The potential for anaphylaxis or other hypersensitivity reactions should be considered based on known hypersensitivity to azithromycin when administered systemically.
Growth of Resistant Organisms with Prolonged Use
- As with other anti-infectives, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If super-infection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and where appropriate, fluorescein staining.
Avoidance of Contact Lenses
- Patients should be advised not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with the rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice.
- The data described below reflect exposure to AzaSite in 698 patients. The population was between 1 and 87 years old with clinical signs and symptoms of bacterial conjunctivitis. The most frequently reported ocular adverse reaction reported in patients receiving AzaSite was eye irritation. This reaction occurred in approximately 1-2% of patients. Other adverse reactions associated with the use of AzaSite were reported in less than 1% of patients and included ocular reactions (blurred vision, burning, stinging and irritation upon instillation, contact dermatitis, corneal erosion, dry eye, eye pain, itching, ocular discharge, punctate keratitis, visual acuity reduction) and non-ocular reactions (dysgeusia, facial swelling, hives, nasal congestion, periocular swelling, rash, sinusitis, urticaria).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Azithromycin (ophthalmic) in the drug label.
Drug Interactions
There is limited information regarding Azithromycin (ophthalmic) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category B. Reproduction studies have been performed in rats and mice at doses up to 200 mg/kg/day. The highest dose was associated with moderate maternal toxicity. These doses are estimated to be approximately 5,000 times the maximum human ocular daily dose of 2 mg. In the animal studies, no evidence of harm to the fetus due to azithromycin was found. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, azithromycin should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Azithromycin (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Azithromycin (ophthalmic) during labor and delivery.
Nursing Mothers
- It is not known whether azithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when azithromycin is administered to a nursing woman.
Pediatric Use
- The safety and effectiveness of AzaSite solution in pediatric patients below 1 year of age have not been established. The efficacy of AzaSite in treating bacterial conjunctivitis in pediatric patients one year or older has been demonstrated in controlled clinical trials
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Azithromycin (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Azithromycin (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Azithromycin (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Azithromycin (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Azithromycin (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Azithromycin (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic
Monitoring
There is limited information regarding Monitoring of Azithromycin (ophthalmic) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Azithromycin (ophthalmic) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Azithromycin (ophthalmic) in the drug label.
Pharmacology
Mechanism of Action
- Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and interfering with microbial protein synthesis.
Structure
- AzaSite (azithromycin ophthalmic solution) is a 1% sterile aqueous topical ophthalmic solution of azithromycin formulated in DuraSite® (polycarbophil, edetate disodium, sodium chloride). AzaSite is an off-white, viscous liquid with an osmolality of approximately 290 mOsm/kg.
- Preservative: 0.003% benzalkonium chloride. Inactives: mannitol, citric acid, sodium citrate, poloxamer 407, polycarbophil, edetate disodium (EDTA), sodium chloride, water for injection, and sodium hydroxide to adjust pH to 6.3.
- Azithromycin is a macrolide antibiotic with a 15-membered ring. Its chemical name is (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyloxy-1-oxa-6-aza-cyclopentadecan-15-one, and the structural formula is:

- Azithromycin has a molecular weight of 749, and its empirical formula is C38H72N2O12.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Azithromycin (ophthalmic) in the drug label.
Pharmacokinetics
- The plasma concentration of azithromycin following ocular administration of AzaSite (azithromycin ophthalmic solution) in humans is unknown. Based on the proposed dose of one drop to each eye (total dose of 100 mcL or 1 mg) and exposure information from systemic administration, the systemic concentration of azithromycin following ocular administration is estimated to be below quantifiable limits (≤10 ng/mL) at steady-state in humans, assuming 100% systemic availability.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in standard laboratory tests: mouse lymphoma assay, human lymphocyte clastogenic assay, and mouse bone marrow clastogenic assay. No evidence of impaired fertility due to azithromycin was found in mice or rats that received oral doses of up to 200 mg/kg/day.
Clinical Studies
- In a randomized, vehicle-controlled, double-blind, multicenter clinical study in which patients were dosed twice daily for the first two days, then once daily on days 3, 4, and 5, AzaSite solution was superior to vehicle on days 6-7 in patients who had a confirmed clinical diagnosis of bacterial conjunctivitis. Clinical resolution was achieved in 63% (82/130) of patients treated with AzaSite versus 50% (74/149) of patients treated with vehicle. The p-value for the comparison was 0.03 and the 95% confidence interval around the 13% (63%-50%) difference was 2% to 25%. The microbiological success rate for the eradication of the baseline pathogens was approximately 88% compared to 66% of patients treated with vehicle (p<0.001, confidence interval around the 22% difference was 13% to 31%). Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
How Supplied
- AzaSite is a sterile aqueous topical ophthalmic formulation of 1% azithromycin.
- NDC 31357-040-25: 2.5 mL in 5 mL bottle containing a total of 25 mg of azithromycin in a white, round, low-density polyethylene (LDPE) bottle, with a clear LDPE dropper tip, and a tan colored high density polyethylene (HDPE) eyedropper cap. A white tamper evident over-cap is provided.
- NDC 31357-040-03: 2.5 mL in 4 mL bottle containing a total of 25 mg of azithromycin in a white, round, low-density polyethylene (LDPE) bottle, with a clear LDPE dropper tip, and a tan colored high density polyethylene (HDPE) eyedropper cap. A white tamper evident over-cap is provided.
Storage
- Store unopened bottle under refrigeration at 2°C to 8°C (36°F to 46°F). Once the bottle is opened, store at 2°C to 25°C (36°F to 77°F) for up to 14 days. Discard after the 14 days.
Images
Drug Images
{{#ask: Page Name::Azithromycin (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Azithromycin (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Azithromycin (ophthalmic) in the drug label.
Precautions with Alcohol
- Alcohol-Azithromycin (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- AZASITE ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "azithromycin monohydrate solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Azithromycin (ophthalmic)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Azithromycin (ophthalmic) |Label Name=Azithromycin (ophthalmic)11.png
}}
{{#subobject:
|Label Page=Azithromycin (ophthalmic) |Label Name=Azithromycin (ophthalmic)11.png
}}