Avatrombopag
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2], Anmol Pitliya, M.B.B.S. M.D.[3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Avatrombopag is a thrombopoietin receptor agonist that is FDA approved for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. Common adverse reactions include pyrexia, abdominal pain, nausea, headache, fatigue, and edema peripheral.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Avatrombopag is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure.
Dosage:
- Begin avatrombopag dosing 10-13 days prior to the scheduled procedure. The recommended daily dose of avatrombopag is based on the patient’s platelet count prior to the scheduled procedure (Refer to Table 1). Patients should undergo their procedure 5 to 8 days after the last dose of avatrombopag.
- Avatrombopag should be taken orally once daily for 5 consecutive days with food. In the case of a missed dose, patients should take the next dose of avatrombopag as soon as they remember. Patients should not take two doses at one time to make up for a missed dose and should take the next dose at the usual time the next day; all five days of dosing should be completed.
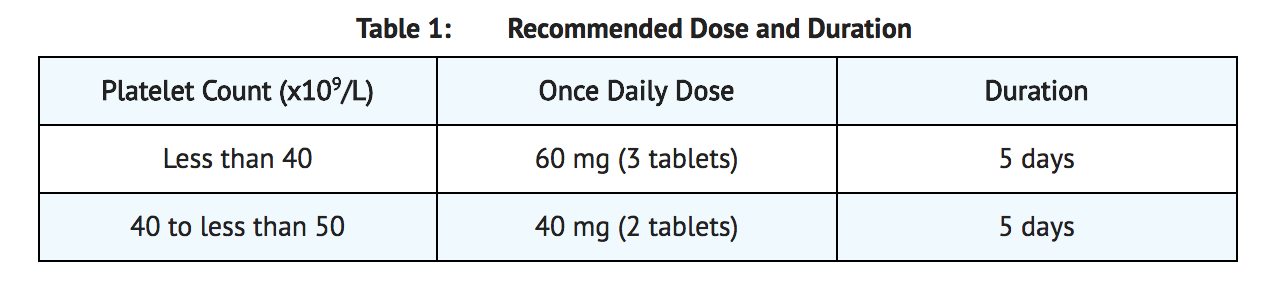
- Avatrombopag has been investigated only as a single 5-day once daily dosing regimen in clinical trials in patients with chronic liver disease. Avatrombopag should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding avatrombopag Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding avatrombopag Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Avatrombopag FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding avatrombopag Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding avatrombopag Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None
Warnings
Thrombotic/Thromboembolic Complications
- Avatrombopag is a thrombopoietin (TPO) receptor agonist and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal vein thrombosis has been reported in patients with chronic liver disease treated with TPO receptor agonists. In the ADAPT-1 and ADAPT-2 clinical trials, there was 1 treatment-emergent event of portal vein thrombosis in a patient (n=1/430) with chronic liver disease and thrombocytopenia treated with avatrombopag. Consider the potential increased thrombotic risk when administering avatrombopag to patients with known risk factors for thromboembolism, including genetic prothrombotic conditions (Factor V Leiden, Prothrombin 20210A, Antithrombin deficiency or Protein C or S deficiency).
- Avatrombopag should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety of avatrombopag was evaluated in two international, identically designed, randomized, double-blind, placebo-controlled trials, ADAPT-1 and ADAPT-2, in which 430 patients with chronic liver disease and thrombocytopenia received either avatrombopag (n=274) or placebo (n=156) daily for 5 days prior to a scheduled procedure, and had 1 post-dose safety assessment. Patients were divided into two groups based on their mean platelet count at baseline:
- Low Baseline Platelet Count Cohort (less than 40 x109/L) who received avatrombopag 60 mg once daily for 5 days.
- High Baseline Platelet Count Cohort (40 to less than 50 x109/ L) who received avatrombopag 40 mg once daily for 5 days.
- The majority of patients were males (65%) and median subject age was 58 years (ranging from 19-86 years of age). The racial and ethnic distribution was White (60%), Asian (33%), Black (3%), and Other (3%).
- The most common adverse reactions (those occurring in ≥3% of patients) in the avatrombopag-treated groups (60 mg or 40 mg) across the pooled data from the two trials are summarized in Table 2.
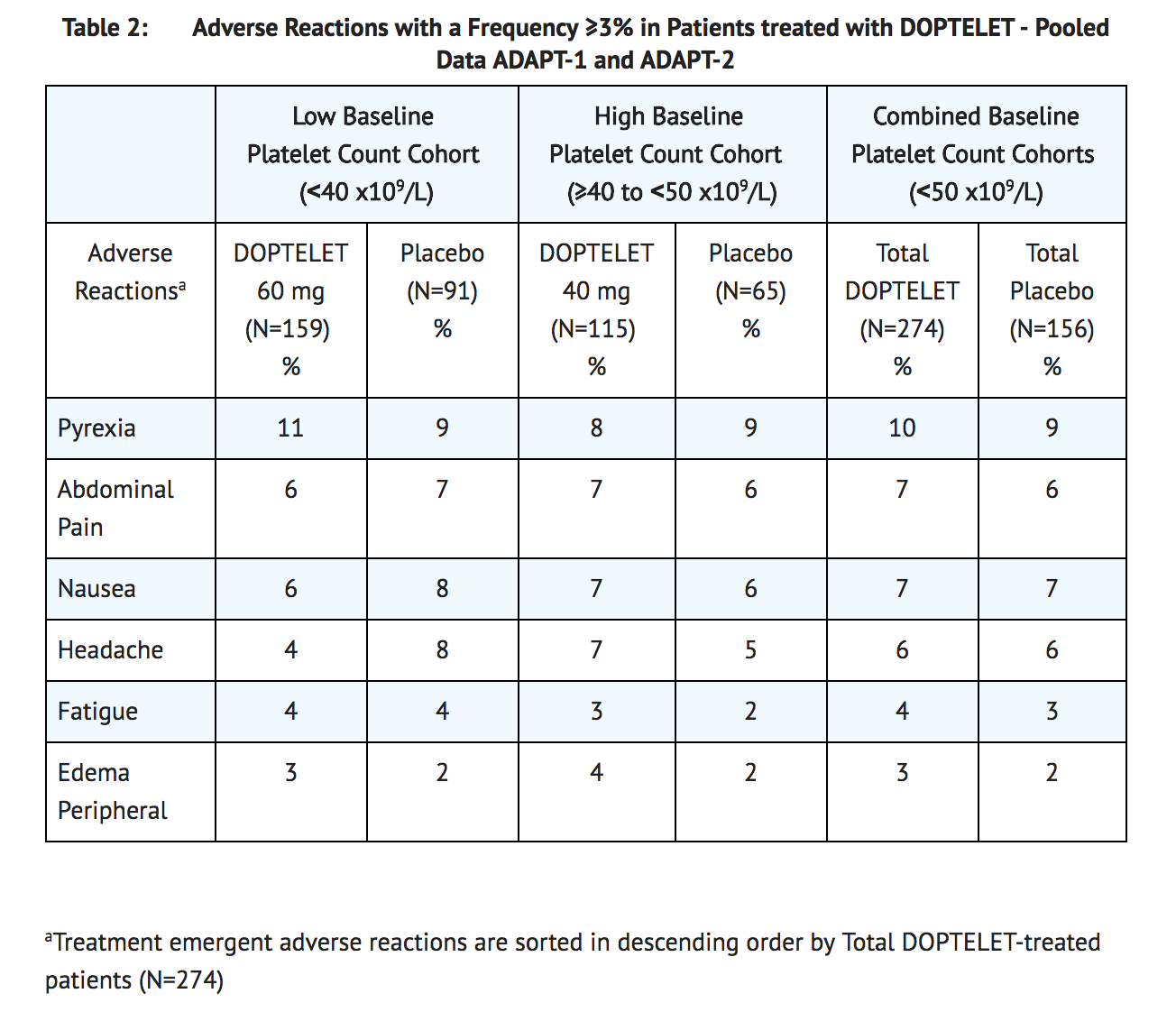
- For the Low Baseline Platelet Count Cohort, the incidence of serious adverse reactions was 7% (11/159) in the 60 mg avatrombopag treatment group and 13% (12/91) in the matching placebo treatment group. For the High Baseline Platelet Count Cohort, the incidence of serious adverse reactions was 8% (9/115) in the 40 mg avatrombopag treatment group and 3% (2/65) in the matching placebo treatment group. The most common serious adverse reaction reported with avatrombopag was hyponatremia. Two avatrombopag-treated patients (0.7%) developed hyponatremia as compared to no patients in the combined placebo group.
- Adverse reactions resulting in discontinuation of avatrombopag were anemia, pyrexia, and myalgia; each was reported in a single (0.4%) patient in the avatrombopag (60 mg) treatment group.
Postmarketing Experience
There is limited information regarding Avatrombopag Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Avatrombopag Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Risk Summary
- Based on findings from animal reproduction studies, avatrombopag may cause fetal harm when administered to a pregnant woman (see Data). The available data on avatrombopag in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. In animal reproduction studies, oral administration of avatrombopag resulted in adverse developmental outcomes when administered during organogenesis in rabbits and during organogenesis and the lactation period in rats. However, these findings were observed at exposures based on AUC substantially higher than the AUC observed in patients at the recommended dose of 60 mg once daily. Advise pregnant women of the potential risk to a fetus.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
- Data (Animal Data)
- In embryo-fetal development studies, avatrombopag was administered during organogenesis at doses of 100, 300, and 1000 mg/kg/day in rats and doses of 100, 300, and 600 mg/kg/day in rabbits. Minimal decreases in fetal weights were observed in rats at the maternally toxic dose of 1000 mg/kg/day with exposures 190-times the human exposure based on AUC. Spontaneous abortions were observed at all doses tested in rabbits and were associated with decreased body weights and food consumption at 300 and 600 mg/kg/day; exposures at the lowest dose of 100 mg/kg/day were 10-times the AUC in patients at the recommended dose of 60 mg once daily. There were no embryo-fetal effects in rats administered avatrombopag at doses up to 100 mg/kg/day (53-times the human exposure based on AUC) or rabbits administered avatrombopag at doses up to 600 mg/kg (35-times the human exposure based on AUC).
- In pre- and postnatal development studies in rats, avatrombopag was administered during both the organogenesis and lactation periods at doses ranging from 5 to 600 mg/kg/day. Doses of 100, 300, and 600 mg/kg/day caused maternal toxicity leading to total litter losses, decreased body weight in pups, and increased pup mortality, with the majority of the pup mortality occurring between postnatal days 14 to 21. At a dose of 50 mg/kg/day that did not produce clear maternal toxicity, avatrombopag caused increased pup mortality from postnatal days 4 to 21, and mortality continued through postnatal day 25. The 50 mg/kg/day dose also decreased body weight gain in the pups, resulting in a delay in sexual maturation. There were no effects on behavioral or reproductive functions in the offspring. The 50 mg/kg/day dose resulted in maternal exposures 43-times and pup exposures approximately 3-times the AUC observed in patients at the recommended dose of 60 mg once daily.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Avatrombopag in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Avatrombopag during labor and delivery.
Nursing Mothers
- Risk Summary
- There are no information regarding the presence of avatrombopag in human milk, the effects on the breastfed child, or the effects on milk production. Avatrombopag was present in the milk of lactating rats. When a drug is present in animal milk, it is likely the drug will be present in human milk. Due to the potential for serious adverse reactions in a breastfed child from avatrombopag, breastfeeding is not recommended during treatment with avatrombopag and for at least 2 weeks after the last dose (see Clinical Considerations).
- Clinical Considerations (Minimizing Exposure)
- A lactating woman should interrupt breastfeeding and pump and discard breastmilk during treatment and for two weeks after the last dose of avatrombopag in order to minimize exposure to a breastfed child.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- Clinical studies of avatrombopag did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Gender
There is no FDA guidance on the use of Avatrombopag with respect to specific gender populations.
Race
There is no FDA guidance on the use of Avatrombopag with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Avatrombopag in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Avatrombopag in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Avatrombopag in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Avatrombopag in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
- Take with food.
- In the case of a missed dose, patients should take the next dose as soon as they remember. Patients should not take 2 doses at one time to make up for a missed dose and should take the next dose at the usual time the next day; all 5 days of dosing should be completed.
Monitoring
- Obtain a platelet count prior to administration of avatrombopag therapy and on the day of a procedure to ensure an adequate increase in platelet count.
- Improvement in platelet counts is indicative of efficacy.
- Platelet counts: Prior to administration and on the day of the procedure.
IV Compatibility
There is limited information regarding the compatibility of Avatrombopag and IV administrations.
Overdosage
- In the event of overdose, platelet count may increase excessively and result in thrombotic or thromboembolic complications. Closely monitor the patient and platelet count. Treat thrombotic complications in accordance with standard of care.
- No antidote for avatrombopag overdose is known.
- Hemodialysis is not expected to enhance the elimination of avatrombopag because avatrombopag is only approximately 6% renally excreted and is highly bound to plasma proteins.
Pharmacology
| |
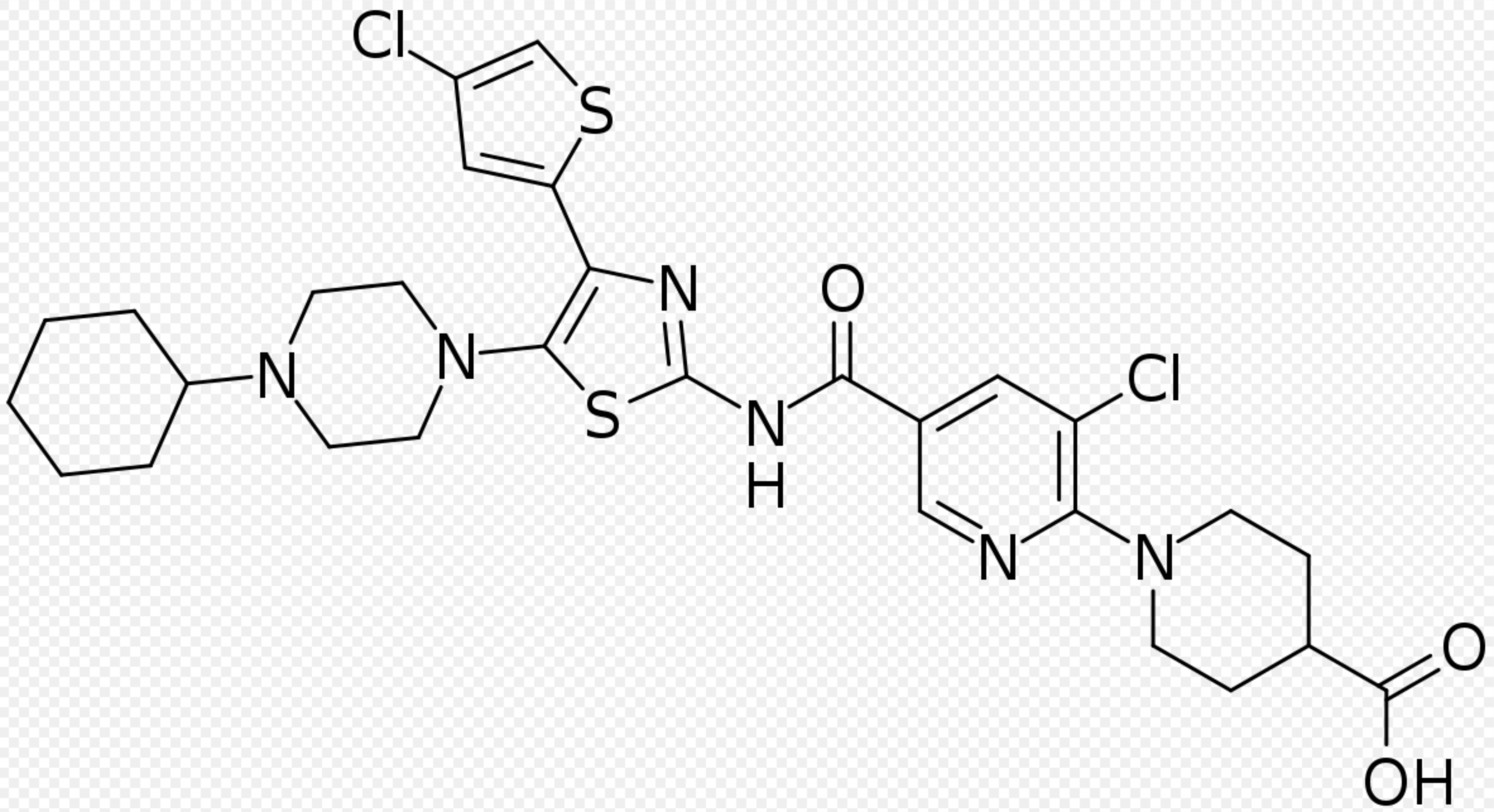
Avatrombopag
| |
| Systematic (IUPAC) name | |
| 1-[3-Chloro-5-[[4-(4-chlorothiophen-2-yl)-5-(4-cyclohexylpiperazin-1-yl)-1,3-thiazol-2-yl]carbamoyl]pyridin-2-yl]piperidine-4-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Oral |
Mechanism of Action
- Avatrombopag is an orally bioavailable, small molecule TPO receptor agonist that stimulates proliferation and differentiation of megakaryocytes from bone marrow progenitor cells resulting in an increased production of platelets. Avatrombopag does not compete with TPO for binding to the TPO receptor and has an additive effect with TPO on platelet production.
Structure
Pharmacodynamics
Platelet Response
- Avatrombopag resulted in dose- and exposure-dependent elevations in platelet counts in adults. The onset of the platelet count increase was observed within 3 to 5 days of the start of a 5-day treatment course, with peak effect observed after 10 to 13 days. Subsequently, platelet counts decreased gradually, returning to near baseline values after 35 days.
Cardiac Electrophysiology
- At exposures similar to that achieved at the 40 mg and 60 mg dose, avatrombopag does not prolong the QT interval to any clinically relevant extent. Mean QTc prolongation effects >20 ms are not anticipated with the highest recommended therapeutic dosing regimen based on analysis of data from the pooled clinical trials in patients with chronic liver disease.
Pharmacokinetics
- Avatrombopag demonstrated dose-proportional pharmacokinetics after single doses from 10 mg (0.25-times the lowest approved dosage) to 80 mg (1.3-times the highest recommended dosage). Healthy subjects administered 40 mg of avatrombopag had a geometric mean (%CV) maximal concentration (Cmax) of 166 (84%) ng/mL and area under the time-concentration curve extrapolated to infinity (AUC0-inf) of 4198 (83%) ng.hr/mL. The pharmacokinetics of avatrombopag were similar in both healthy subjects and the chronic liver disease population.
Absorption
- The median time to maximal concentration (Tmax) occurred at 5 to 6 hours post-dose.
Effect of Food
- Avatrombopag AUC0-inf and Cmax were not affected when avatrombopag was co-administered with a low-fat meal (500 calories, 3 g fat, 15 g proteins, and 108 g carbohydrates) or a high-fat meal (918 calories, 59 g fat, 39 g proteins, and 59 g carbohydrates). The variability of avatrombopag exposure was reduced by 40% to 60% with food. The Tmax of avatrombopag was delayed by 0 to 2 hours when avatrombopag was administered with a low-fat or high-fat meal (median Tmax range 5 to 8 hours) compared to the fasted state.
Distribution
- Avatrombopag has an estimated mean volume of distribution (%CV) of 180 L (25%). Avatrombopag is greater than 96% bound to human plasma proteins.
Elimination
- The mean plasma elimination half-life (%CV) of avatrombopag is approximately 19 hours (19%). The mean (%CV) of the clearance of avatrombopag is estimated to be 6.9 L/hr (29%).
Metabolism
- Avatrombopag is primarily metabolized by cytochrome P450 (CYP) 2C9 and CYP3A4.
Excretion
- Fecal excretion accounted for 88% of the administered dose, with 34% of the dose excreted as unchanged avatrombopag. Only 6% of the administered dose was found in urine.
Specific Populations
- Age (18-86 years), body weight (39-175 kg), sex, race [Whites, African Americans, and East Asians (i.e., Japanese, Chinese and Koreans)], and any hepatic impairment (Child-Turcotte-Pugh (CTP) grade A, B, and C, or Model for End-Stage Liver Disease (MELD) score 4-23) and mild to moderate renal impairment (CLcr ≥30 mL/min) did not have clinically meaningful effects on the pharmacokinetics of avatrombopag.
- The effect of age (< 18 years) and severe renal impairment (CLcr < 30 mL/min, Cockcroft-Gault) including patients requiring hemodialysis on avatrombopag pharmacokinetics is unknown.
Drug Interactions
- Drug interaction studies were performed in healthy subjects with single 20 mg avatrombopag dose and drugs likely to be co-administered or drugs commonly used as probes for pharmacokinetic interactions (see Table 3).
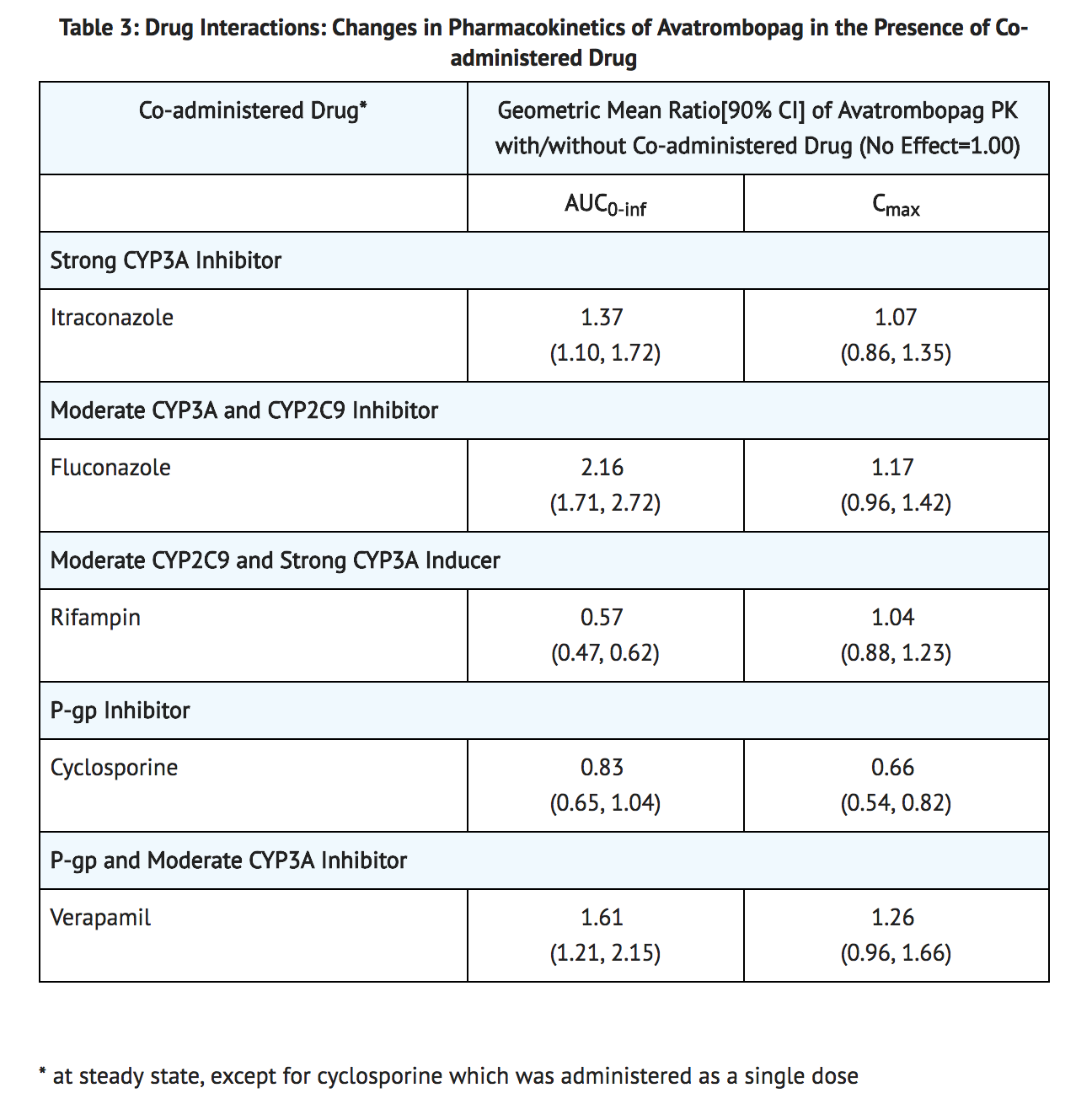
Effect of Avatrombopag
- Avatrombopag does not inhibit CYP1A, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, or CYP3A, does not induce CYP1A, CYP2B6, CYP2C, and CYP3A, and weakly induces CYP2C8 and CYP2C9 in vitro.
- Avatrombopag inhibits organic anion transporter (OAT) 3 and breast cancer resistance protein (BCRP) but not organic anion transporter polypeptide (OATP) 1B1 and 1B3, organic cation transporter (OCT) 2, and OAT1 in vitro.
Effect of Transporters
- Avatrombopag is a substrate for P-glycoprotein (P-gp) mediated transport [see Table 3]. Avatrombopag is not a substrate for OATP1B1, OATP1B3, OCT2, OAT1, and OAT3.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In two-year carcinogenicity studies, avatrombopag was administered orally at doses of 20, 60, 160 mg/kg/day in mice and doses of 20, 50, 160 mg/kg/day in rats. Avatrombopag induced a statistically significant increase in neuroendocrine cell (enterochromaffin-like cell, ECL cell) gastric tumors (carcinoids) in the stomach at 160 mg/kg in female rats. The 160 mg/kg/day dose resulted in exposures 117-times the AUC observed in patients at the recommended dose of 60 mg once daily. The gastric carcinoids were considered likely due to prolonged hypergastrinemia observed in toxicity studies. Hypergastrinemia-related gastric carcinoids in rodents are generally considered to be of low risk or relevance to humans.
- Avatrombopag was not mutagenic in an in vitro bacterial reverse mutation (AMES) assay or clastogenic in an in vitro human lymphocyte chromosomal aberrations assay or in an in vivo rat bone marrow micronucleus assay.
- Avatrombopag did not affect fertility or early embryonic development in male rats at exposures 22-times, or in female rats at exposures 114-times, the AUC observed in patients at the recommended dose of 60 mg once daily.
Clinical Studies
- The efficacy of avatrombopag for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a procedure was established in 2 identically-designed multicenter, randomized, double-blind, placebo-controlled trials (ADAPT-1 (NCT01972529) and ADAPT-2 (NCT01976104)). In each study, patients were assigned to the Low Baseline Platelet Count Cohort (˂40 x109L) or the High Baseline Platelet Count Cohort (≥40 to ˂50 x109 L) based on their platelet count at Baseline. Patients were then randomized in a 2:1 ratio to either avatrombopag or placebo. Patients were stratified according to hepatocellular cancer (HCC) status and risk of bleeding associated with the elective procedure (low, moderate, or high). Patients undergoing neurosurgical interventions, thoracotomy, laparotomy or organ resection were not eligible for enrollment.
- Patients in the Low Baseline Platelet Count Cohort received 60 mg avatrombopag or matching placebo once daily for 5 days, and patients in the High Baseline Platelet Count Cohort received 40 mg avatrombopag or matching placebo once daily for 5 days. Eligible patients were scheduled to undergo their procedure (low, moderate, or high bleeding risk) 5 to 8 days after their last dose of treatment. Patient populations were similar between the pooled Low and High Baseline Platelet Count Cohorts and consisted of 66% male and 35% female; median age 58 years and 61% White, 34% Asian, and 3% Black.
- In ADAPT-1, a total of 231 patients were randomized, 149 patients were treated with avatrombopag and 82 patients were treated with placebo. In the Low Baseline Platelet Count Cohort, the mean Baseline platelet count for the avatrombopag-treated group was 31.1 x109/L and for placebo-treated patients was 30.7 x109/L. In the High Baseline Platelet Count Cohort, the mean Baseline platelet count for the avatrombopag-treated patients was 44.3 x109/L and for placebo-treated patients was 44.9 x109/L.
- In ADAPT-2, a total of 204 patients were randomized, 128 patients were treated with avatrombopag and 76 patients were treated with placebo. In the Low Baseline Platelet Count Cohort, the mean Baseline platelet count for the avatrombopag-treated group was 32.7 x109/L and for placebo-treated patients was 32.5 x109/L. In the High Baseline Platelet Count Cohort, the mean Baseline platelet count for the avatrombopag-treated patients was 44.3 x109/L and for placebo-treated patients was 44.5 x109/L.
- Across both baseline platelet count cohorts and the avatrombopag and placebo treatment groups, patients underwent a broad spectrum of types of scheduled procedures that ranged from low to high bleeding risk. Overall, the majority of patients (60.8% [248/430] subjects) in all treatment groups underwent low bleeding risk procedures, 17.2% [70/430] of patients underwent procedures associated with moderate bleeding risk, and 22.1% [90/430] of subjects underwent procedures associated with high bleeding risk. The proportions of patients undergoing low, moderate, and high-risk procedures were similar between the avatrombopag and placebo treatment groups.
- The major efficacy outcome was the proportion of patients who did not require a platelet transfusion or any rescue procedure for bleeding after randomization and up to 7 days following an elective procedure. Additional secondary efficacy outcomes were the proportion of patients who achieved platelet counts of >50 x109/L on the day of procedure and the change in platelet count from baseline to procedure day.
- Responders were defined as patients who did not require a platelet transfusion or any rescue procedure for bleeding after randomization and up to 7 days following a scheduled procedure. The following were considered rescue therapies to manage risk of bleeding associated with a procedure: whole blood transfusion, packed red blood cell (RBC) transfusion, platelet transfusion, fresh frozen plasma (FFP) or cryoprecipitate administration, Vitamin K, desmopressin, recombinant activated factor VII, aminocaproic acid, tranexamic acid, or surgical or interventional radiology procedures performed to achieve hemostasis and control blood loss. In both baseline platelet count cohorts, patients in the avatrombopag treatment groups had a greater proportion of responders than the corresponding placebo treatment groups that was both clinically meaningful and statistically significant as detailed in Table 4.
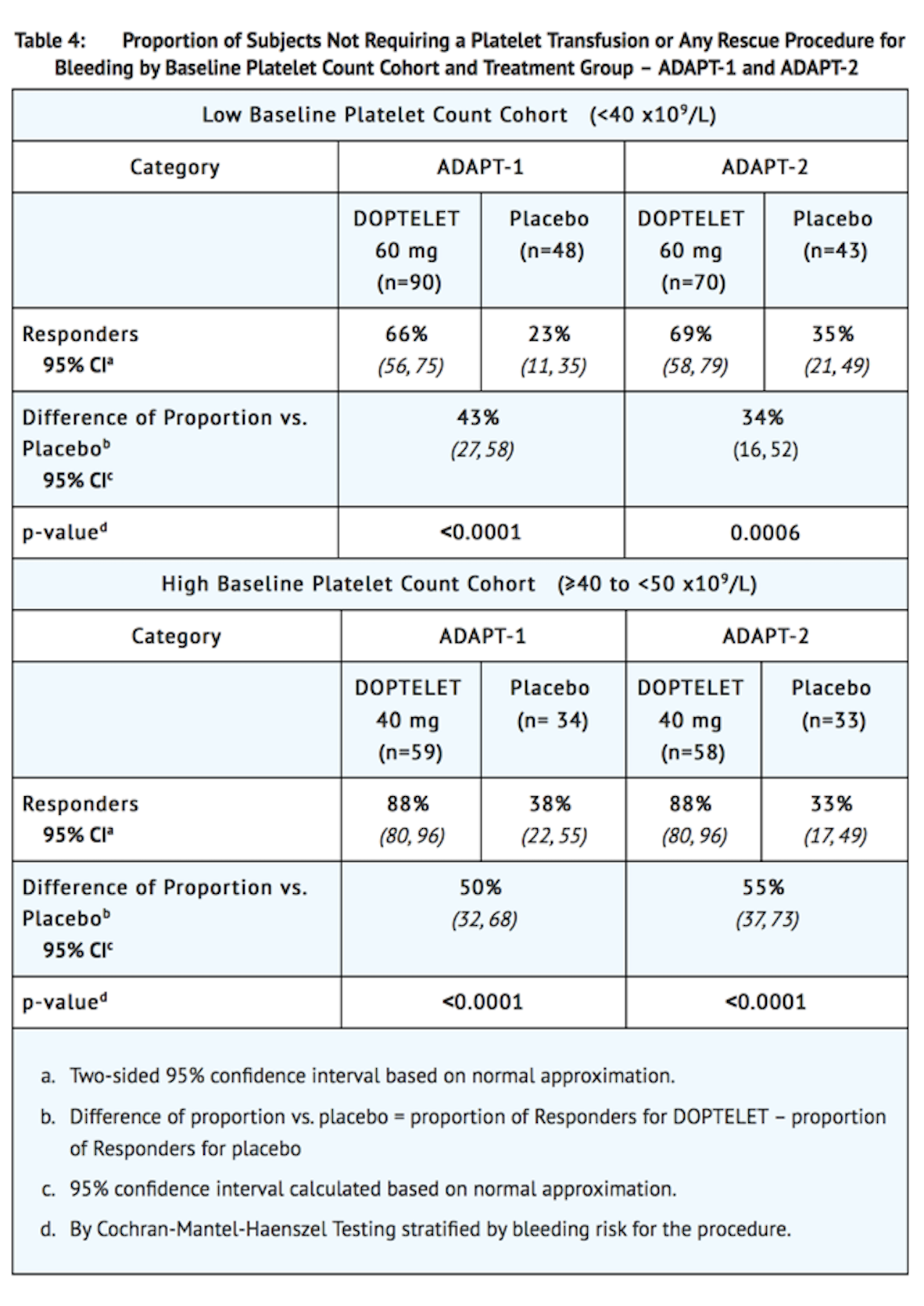
- In addition, both trials demonstrated a higher proportion of patients who achieved the target platelet count of ≥ 50 x109/L on the day of the procedure, a secondary efficacy endpoint, in both avatrombopag-treated groups versus the placebo-treated groups for both cohorts (Low Baseline Platelet Count Cohort-ADAPT-1: 69% vs 4%, respectively; P <0.0001; ADAPT-2: 67% vs 7%, respectively; P <0.0001; High Baseline Platelet Count Cohort- ADAPT-1: 88% vs 21%, respectively; P <0.0001; ADAPT-2: 93% vs 39%, respectively; P <0.0001).
- Further, both trials demonstrated a greater mean change in platelet counts from baseline to the day of the procedure, a secondary efficacy endpoint, in both avatrombopag-treated groups versus the placebo-treated groups for both cohorts (Low Baseline Platelet Count Cohort-ADAPT-1: 32 x109/L vs 0.8 x109/L, respectively; P <0.0001; ADAPT-2: 31.3 x109/L vs 3.0 x109/L, respectively; P <0.0001; High Baseline Platelet Count Cohort-ADAPT-1: 37.1 x109/L vs 1.0 x109/L, respectively; P <0.0001;ADAPT-2: 44.9 x109/L vs 5.9 x109/L, respectively; P <0.0001).
- A measured increase in platelet counts was observed in both avatrombopag treatment groups over time beginning on Day 4 post-dose, that peaked on Day 10-13, decreased 7 days post-procedure, and then returned to near baseline values by Day 35.
How Supplied
- Avatrombopag 20 mg tablets are supplied as round, biconvex, yellow, film-coated tablets, and debossed with “AVA” on one side and “20” on the other side.
- NDC 71369-020-10: carton with one blister card of ten 20 mg tablets.
- NDC 71369-020-11: one blister card with ten 20 mg tablets.
- NDC 71369-020-15: carton with one blister card of fifteen 20 mg tablets.
- NDC 71369-020-16: one blister card of fifteen 20 mg tablets.
Storage
- Store at 20°C to 25°C (68°F to 77°F), excursions permitted to 15°C to 30°C (59°F to 86°F). Store tablets in original package.
Images
Drug Images
{{#ask: Page Name::Avatrombopag |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Avatrombopag |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Risks
Thrombotic/Thromboembolic Complications
- Avatrombopag is a thrombopoietin (TPO) receptor agonist and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal vein thrombosis has been reported in patients with chronic liver disease treated with TPO receptor agonists.
Pregnancy
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy.
Lactation
- Advise women not to breastfeed during treatment with avatrombopag and for at least 2 weeks after the final dose.
Precautions with Alcohol
Alcohol-Avatrombopag interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Doptelet
Look-Alike Drug Names
There is limited information regarding Avatrombopag Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.