Atrial septal defect pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Template:Atrial septal defect}} | {{Template:Atrial septal defect}} | ||
{{CMG}} | {{CMG}}; Claudia Hochberg, M.D. | ||
'''Associate Editors-In-Chief:''' {{CZ}}; [[User:KeriShafer|Keri Shafer, M.D.]] [mailto:kshafer@bidmc.harvard.edu]; [[Priyamvada Singh|Priyamvada Singh, MBBS]] [[mailto:psingh@perfuse.org]] | |||
'''Assistant Editor-In-Chief:''' [[Kristin Feeney|Kristin Feeney, B.S.]] [[mailto:kfeeney@perfuse.org]] | |||
==Overview== | |||
In a normal heart, the typical path of blood flow is to shunt from right-to-left allowing for blood to leave the deoxygenated right system and become oxygenated in the left system. In patients with an atrial septal defect, the typical path of blood flow is disrupted by the septal opening. This results in blood shunting from [[atrial septal defect left-to-right shunt|left-to-right]]. The severity of circulatory complications depends largely on the size of the defect. The larger the defect between the septal walls, the greater the amount of mixing between deoxygenated and oxygenated blood. | |||
==Pathophysiology== | ==Pathophysiology== | ||
In unaffected individuals, the chambers of the left side of the heart make up a higher pressure system than the chambers of the right side of the heart. This is because the [[left ventricle]] has to produce enough pressure to pump blood throughout the entire body, while the [[right ventricle]] only has to produce enough pressure to pump blood to the [[lung]]s. | In unaffected individuals, the chambers of the left side of the heart make up a higher pressure system than the chambers of the right side of the heart. This is because the [[left ventricle]] has to produce enough pressure to pump blood throughout the entire body, while the [[right ventricle]] only has to produce enough pressure to pump blood to the [[lung]]s. | ||
In the case of a large [[ASD]] (>9mm), which may result in a clinically remarkable ''[[left-to-right shunt]]'', blood will shunt from the [[left atrium]] to the [[right atrium]] causing excessive interatrial communication (In the case of hemodynamically significant [[ASD]] Qp is pulmanry flow and Qs is systemic flow and the Qp:Qs > 1.5:1), the patient is often symptomatic and [[ASD]] repair may be indicated. This extra blood from the left atrium may cause a volume overload of both the [[right atrium]] and the [[right ventricle]], which if left untreated, can result in enlargement of the right side of the heart and ultimately [[heart failure]]. | In the case of a large [[ASD]] (>9mm), which may result in a clinically remarkable ''[[atrial septal defect left-to-right shunt|left-to-right shunt]]'', blood will shunt from the [[left atrium]] to the [[right atrium]] causing excessive interatrial communication (In the case of hemodynamically significant [[ASD]] Qp is pulmanry flow and Qs is systemic flow and the Qp:Qs > 1.5:1), the patient is often symptomatic and [[ASD]] repair may be indicated. This extra blood from the left atrium may cause a volume overload of both the [[right atrium]] and the [[right ventricle]], which if left untreated, can result in enlargement of the right side of the heart and ultimately [[heart failure]]. | ||
Any process that increases the pressure in the [[left ventricle]] can cause worsening of the left-to-right shunt. This includes [[hypertension]], which increases the pressure that the [[left ventricle]] has to generate in order to open the [[aortic valve]] during ventricular [[systole]], and [[coronary artery disease]] which increases the stiffness of the [[left ventricle]], thereby increasing the filling pressure of the left ventricle during ventricular [[diastole]]. | Any process that increases the pressure in the [[left ventricle]] can cause worsening of the left-to-right shunt. This includes [[hypertension]], which increases the pressure that the [[left ventricle]] has to generate in order to open the [[aortic valve]] during ventricular [[systole]], and [[coronary artery disease]] which increases the stiffness of the [[left ventricle]], thereby increasing the filling pressure of the left ventricle during ventricular [[diastole]]. | ||
| Line 21: | Line 28: | ||
==Pathological Findings== | ==Pathological Findings== | ||
[http://www.peir.net Images courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | [http://www.peir.net Images courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] | ||
<div align="left"> | <div align="left"> | ||
<gallery> | |||
<gallery | |||
Image:Congenital heart defect 0053.jpg|Atrial Septal Defect, Septum Primum; View from Right Atrium (a 4 month old baby) | Image:Congenital heart defect 0053.jpg|Atrial Septal Defect, Septum Primum; View from Right Atrium (a 4 month old baby) | ||
Image:Congenital heart defect 0054.jpg|Atrial Septal Defect, Septum Primum; Also Cleft in Anterior Cusp of Mitral Valve | Image:Congenital heart defect 0054.jpg|Atrial Septal Defect, Septum Primum; Also Cleft in Anterior Cusp of Mitral Valve | ||
Revision as of 20:16, 25 July 2011
|
Atrial Septal Defect Microchapters | |
|
Treatment | |
|---|---|
|
Surgery | |
|
| |
|
Special Scenarios | |
|
Case Studies | |
|
Atrial septal defect pathophysiology On the Web | |
|
American Roentgen Ray Society Images of Atrial septal defect pathophysiology | |
|
Risk calculators and risk factors for Atrial septal defect pathophysiology | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Claudia Hochberg, M.D.
Associate Editors-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Keri Shafer, M.D. [3]; Priyamvada Singh, MBBS [[4]]
Assistant Editor-In-Chief: Kristin Feeney, B.S. [[5]]
Overview
In a normal heart, the typical path of blood flow is to shunt from right-to-left allowing for blood to leave the deoxygenated right system and become oxygenated in the left system. In patients with an atrial septal defect, the typical path of blood flow is disrupted by the septal opening. This results in blood shunting from left-to-right. The severity of circulatory complications depends largely on the size of the defect. The larger the defect between the septal walls, the greater the amount of mixing between deoxygenated and oxygenated blood.
Pathophysiology
In unaffected individuals, the chambers of the left side of the heart make up a higher pressure system than the chambers of the right side of the heart. This is because the left ventricle has to produce enough pressure to pump blood throughout the entire body, while the right ventricle only has to produce enough pressure to pump blood to the lungs.
In the case of a large ASD (>9mm), which may result in a clinically remarkable left-to-right shunt, blood will shunt from the left atrium to the right atrium causing excessive interatrial communication (In the case of hemodynamically significant ASD Qp is pulmanry flow and Qs is systemic flow and the Qp:Qs > 1.5:1), the patient is often symptomatic and ASD repair may be indicated. This extra blood from the left atrium may cause a volume overload of both the right atrium and the right ventricle, which if left untreated, can result in enlargement of the right side of the heart and ultimately heart failure.
Any process that increases the pressure in the left ventricle can cause worsening of the left-to-right shunt. This includes hypertension, which increases the pressure that the left ventricle has to generate in order to open the aortic valve during ventricular systole, and coronary artery disease which increases the stiffness of the left ventricle, thereby increasing the filling pressure of the left ventricle during ventricular diastole.
The right ventricle will have to push out more blood than the left ventricle due to the left-to-right shunt. This constant overload of the right side of the heart will cause an overload of the entire pulmonary vasculature. Eventually the pulmonary vasculature will develop pulmonary hypertension as a result of the extra blood flow through the lungs.
The pulmonary hypertension will cause the right ventricle to face increased pressure of afterload in addition to the increased preload (higher volume coming into the right side of the heart) as a result of the shunted blood flowing from the left atrium into the right atrium. The right ventricle will be forced to generate higher pressures to try to overcome the pulmonary hypertension. This may lead to right ventricular failure (dilatation and decreased systolic function of the right ventricle). Eventually the right-sided pressures may exceed left-sided pressures.
When the pressure in the right atrium rises to the level in the left atrium, there will no longer be a pressure gradient between these heart chambers, and the left-to-right shunt will diminish or cease.
If left uncorrected, the pressure in the right side of the heart will be greater than the left side of the heart. This will cause the pressure in the right atrium to be higher than the pressure in the left atrium. This will reverse the pressure gradient across the ASD, and the shunt will reverse; a right-to-left shunt will exist. This phenomenon is known as Eisenmenger's syndrome.
Once right-to-left shunting occurs, a portion of the oxygen-poor blood will get shunted to the left side of the heart and ejected to the peripheral vascular system. This will cause signs of cyanosis.
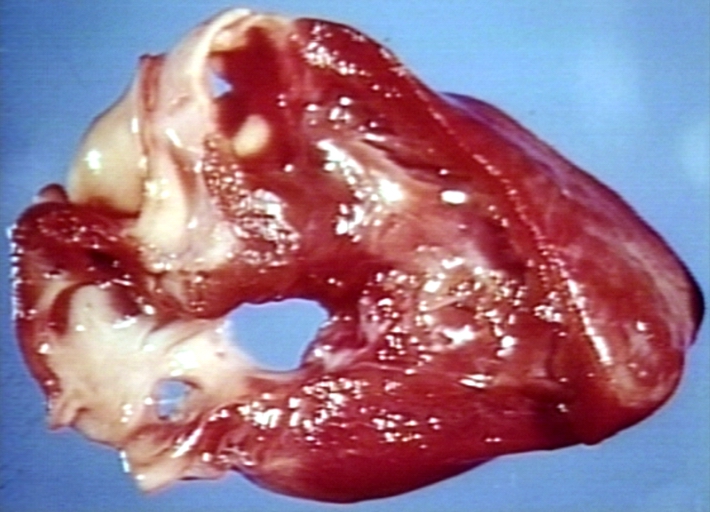
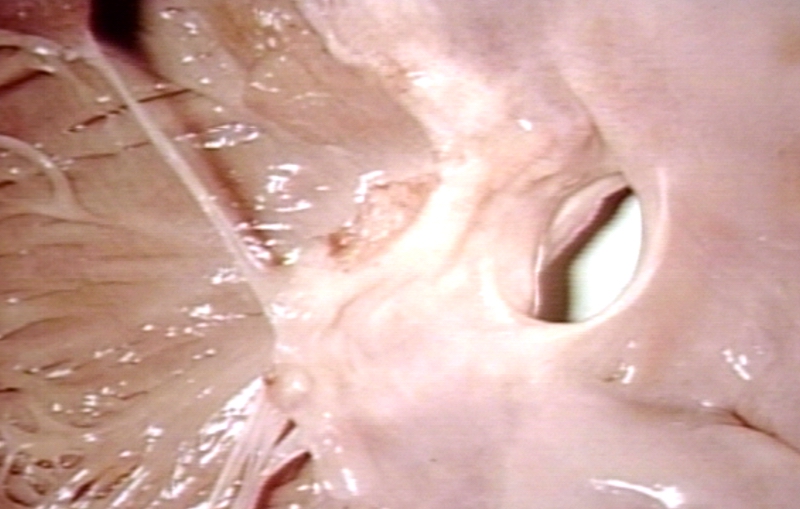
Pathological Findings
-
Atrial Septal Defect, Septum Primum; View from Right Atrium (a 4 month old baby)
-
Atrial Septal Defect, Septum Primum; Also Cleft in Anterior Cusp of Mitral Valve