Ataxin 3
| Ataxin 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1yzb. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | ATXN3 ; AT3; ATX3; JOS; MJD; MJD1; SCA3 | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 3658 | ||||||||||||
| |||||||||||||
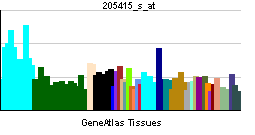
| RNA expression pattern | |||||||||||||
 | |||||||||||||
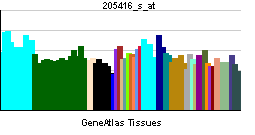 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Ataxin 3, also known as ATXN3, is a human gene.[1]
Machado-Joseph disease, also known as spinocerebellar ataxia-3, is an autosomal dominant neurologic disorder. The protein encoded by this gene contains (CAG)n repeats in the coding region, and the expansion of these repeats from the normal 13-36 to 68-79 is the cause of Machado-Joseph disease. There is a negative correlation between the age of onset and CAG repeat numbers. Alternatively spliced transcript variants encoding different isoforms have been described for this gene.[2]
References
Further reading
- Goto J, Watanabe M, Ichikawa Y; et al. (1997). "Machado-Joseph disease gene products carrying different carboxyl termini". Neurosci. Res. 28 (4): 373–7. PMID 9274833.
- Schöls L, Vieira-Saecker AM, Schöls S; et al. (1995). "Trinucleotide expansion within the MJD1 gene presents clinically as spinocerebellar ataxia and occurs most frequently in German SCA patients". Hum. Mol. Genet. 4 (6): 1001–5. PMID 7655453.
- Stevanin G, Cancel G, Dürr A; et al. (1995). "The gene for spinal cerebellar ataxia 3 (SCA3) is located in a region of approximately 3 cM on chromosome 14q24.3-q32.2". Am. J. Hum. Genet. 56 (1): 193–201. PMID 7825578.
- Kawaguchi Y, Okamoto T, Taniwaki M; et al. (1995). "CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1". Nat. Genet. 8 (3): 221–8. doi:10.1038/ng1194-221. PMID 7874163.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. PMID 8125298.
- Takiyama Y, Nishizawa M, Tanaka H; et al. (1993). "The gene for Machado-Joseph disease maps to human chromosome 14q". Nat. Genet. 4 (3): 300–4. doi:10.1038/ng0793-300. PMID 8358439.
- Ikeda H, Yamaguchi M, Sugai S; et al. (1996). "Expanded polyglutamine in the Machado-Joseph disease protein induces cell death in vitro and in vivo". Nat. Genet. 13 (2): 196–202. doi:10.1038/ng0696-196. PMID 8640226.
- Paulson HL, Das SS, Crino PB; et al. (1997). "Machado-Joseph disease gene product is a cytoplasmic protein widely expressed in brain". Ann. Neurol. 41 (4): 453–62. doi:10.1002/ana.410410408. PMID 9124802.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K; et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. PMID 9373149.
- Wellington CL, Ellerby LM, Hackam AS; et al. (1998). "Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract". J. Biol. Chem. 273 (15): 9158–67. PMID 9535906.
- Tait D, Riccio M, Sittler A; et al. (1998). "Ataxin-3 is transported into the nucleus and associates with the nuclear matrix". Hum. Mol. Genet. 7 (6): 991–7. PMID 9580663.
- Wang G, Sawai N, Kotliarova S; et al. (2000). "Ataxin-3, the MJD1 gene product, interacts with the two human homologs of yeast DNA repair protein RAD23, HHR23A and HHR23B". Hum. Mol. Genet. 9 (12): 1795–803. PMID 10915768.
- Ichikawa Y, Goto J, Hattori M; et al. (2001). "The genomic structure and expression of MJD, the Machado-Joseph disease gene". J. Hum. Genet. 46 (7): 413–22. PMID 11450850.
- Chai Y, Shao J, Miller VM; et al. (2002). "Live-cell imaging reveals divergent intracellular dynamics of polyglutamine disease proteins and supports a sequestration model of pathogenesis". Proc. Natl. Acad. Sci. U.S.A. 99 (14): 9310–5. doi:10.1073/pnas.152101299. PMID 12084819.
- Yoshida H, Yoshizawa T, Shibasaki F; et al. (2002). "Chemical chaperones reduce aggregate formation and cell death caused by the truncated Machado-Joseph disease gene product with an expanded polyglutamine stretch". Neurobiol. Dis. 10 (2): 88–99. PMID 12127147.
- Li F, Macfarlan T, Pittman RN, Chakravarti D (2003). "Ataxin-3 is a histone-binding protein with two independent transcriptional corepressor activities". J. Biol. Chem. 277 (47): 45004–12. doi:10.1074/jbc.M205259200. PMID 12297501.
- Strausberg RL, Feingold EA, Grouse LH; et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMID 12477932.
- Albrecht M, Hoffmann D, Evert BO; et al. (2003). "Structural modeling of ataxin-3 reveals distant homology to adaptins". Proteins. 50 (2): 355–70. doi:10.1002/prot.10280. PMID 12486728.
- Marchal S, Shehi E, Harricane MC; et al. (2003). "Structural instability and fibrillar aggregation of non-expanded human ataxin-3 revealed under high pressure and temperature". J. Biol. Chem. 278 (34): 31554–63. doi:10.1074/jbc.M304205200. PMID 12766160.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |