Articaine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Articaine is a amide local anesthetic that is FDA approved for the procedure of local, infiltrative, or conductive anesthesia in both simple and complex dental procedures. Common adverse reactions include headache and pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Articadent, an amide local anesthetic containing a vasoconstrictor, is indicated for local, infiltrative, or conductive anesthesia in both simple and complex dental procedures.
General Dosing Information
- Table 1 (below) summarizes the recommended volumes and concentrations of Articadent for various types of anesthetic procedures. The dosages suggested in this table are for normal healthy adults, administered by submucosal infiltration or nerve block.
- The recommended doses serve only as a guide to the amount of anesthetic required for most routine procedures. The actual volumes to be used depend on a number of factors such as type and extent of surgical procedure, depth of anesthesia, degree of muscular relaxation, and condition of the patient. In all cases, the smallest dose that will produce the desired result should be given.
- The onset of anesthesia, and the duration of anesthesia are proportional to the volume and concentration (i.e., total dose) of local anesthetic used. Caution should be exercised when employing large volumes because the incidence of side effects may be dose-related.
- For most routine dental procedures, Articadent containing epinephrine 1:200,000 is preferred. However, when more pronounced hemostasis or improved visualization of the surgical field are required, Articadent containing epinephrine 1:100,000 may be used.
Maximum Recommended Dosages
- Adults: For normal healthy adults, the maximum dose of articaine HCl administered by submucosal infiltration and/or nerve block should not exceed 7 mg/kg (0.175 mL/kg).
- Pediatric Patients Ages 4 to 16 Years: The quantity of articaine HCl in children ages 4 to 16 years of age to be injected should be determined by the age and weight of the child and the magnitude of the operation. The maximum dose of articaine HCl should not exceed 7 mg/kg (0.175 mL/kg).
- Safety and effectiveness of Articadent in pediatric patients below the age of 4 years have not been established.
Dosing in Special Populations
- Dose reduction may be required in debilitated patients, acutely ill patients, elderly patients, and pediatric patients commensurate with their age and physical condition. No studies have been performed in patients with renal or liver dysfunction. Caution should be used in patients with severe liver disease
DOSAGE FORMS AND STRENGTHS
- Injection (clear colorless solution) containing:
- Articaine hydrochloride 4% (40 mg/mL) and epinephrine 1:200,000 (as epinephrine bitartrate 0.009 mg/mL)
- Articaine hydrochloride 4% (40 mg/mL) and epinephrine 1:100,000 (as epinephrine bitartrate 0.018 mg/mL)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Articaine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Articaine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Articaine in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Articaine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Articaine in pediatric patients.
Contraindications
- Articadent is contraindicated in patients who are hypersensitive to products containing sulfites. Products containing sulfites may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episode in certain susceptible people. Sulfite sensitivity is seen more frequently in asthmatic that in non-asthmatic people
Warnings
Accidental Intravascular Injection
- Accidental intravascular injection of Articadent may be associated with convulsions, followed by central nervous system or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Dental practitioners who employ local anesthetic agents including Articadent should be well versed in diagnosis and management of emergencies that may arise from their use. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use. To avoid intravascular injection, aspiration should be performed before Articadent is injected. The needle must be repositioned until no return of blood can be elicited by aspiration. Note, however, that the absence of blood in the syringe does not guarantee that intravascular injection has been avoided.
- Small doses of local anesthetics injected in dental blocks may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. Confusion, convulsions, respiratory depression or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. Patients receiving these blocks should be observed constantly. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded.
Systemic Toxicity
- This includes toxicity arising from accidental intravascular injection of Articadent discussed in Section 5.1, as well as that related to higher systemic concentrations of local anesthetics or epinephrine. Systemic absorption of local anesthetics including Articadent can produce effects on the central nervous and cardiovascular systems.
- At blood concentrations achieved with therapeutic doses of Articadent, changes in cardiac conduction, excitability, refractoriness, contractility, and peripheral vascular resistance are minimal. However, toxic blood concentrations of Articadent can depress cardiac conduction and excitability, which may lead to atrioventricular block, ventricular arrhythmias, and cardiac arrest, possibly resulting in fatalities. In addition, myocardial contractility is depressed and peripheral vasodilation occurs, leading to decreased cardiac output and arterial blood pressure. Articadent should also be used with caution in patients with heart block as well as those with impaired cardiovascular function since they may be less able to compensate for functional changes associated with the prolongation of A-V conduction produced by these drugs.
- Restlessness, anxiety, tinnitus, dizziness, blurred vision, tremors, depression, or drowsiness may be early warning signs of central nervous system toxicity.
- Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient's state of consciousness should be performed after each local anesthetic injection of Articadent. Repeated doses of Articadent may cause significant increases in blood levels because of possible accumulation of the drug or its metabolites. The lowest dosage that results in effective anesthesia should be used to decrease the risk of high plasma levels and serious adverse effects. Tolerance to elevated blood levels varies with the status of the patient. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use. Precautions for epinephrine administration, discussed in Section 5.3 should be observed.
- Debilitated patients, elderly patients, acutely ill patients, and pediatric patients should be given reduced doses commensurate with their age and physical condition. No studies have been performed in patients with liver dysfunction, and caution should be used in patients with severe hepatic disease.
Vasoconstrictor Toxicity
- Articadent contains epinephrine, a vasoconstrictor that can cause local or systemic toxicity and should be used cautiously. Local toxicity may include ischemic injury or necrosis, which may be related to vascular spasm. Articadent should be used with caution in patients during or following the administration of potent general anesthetic agents, since cardiac arrhythmias may occur under such conditions. Patients with peripheral vascular disease and those with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response.
- The American Heart Association has made the following recommendation regarding the use of local anesthetics with vasoconstrictors in patients with ischemic heart disease: "Vasoconstrictor agents should be used in local anesthesia solutions during dental practice only when it is clear that the procedure will be shortened or the analgesia rendered more profound. When a vasoconstrictor is indicated, extreme care should be taken to avoid intravascular injection. The minimum possible amount of vasoconstrictor should be used." (Kaplan, 1986).
- It is essential to aspirate before any injection to avoid administration of the drug into the blood stream.
Methemoglobinemia
- Articadent, like other local anesthetics, can cause methemoglobinemia, particularly in conjunction with methemoglobin-inducing agents. Articadent should not be used in patients with congenital or idiopathic methemoglobinemia, or in patients who are receiving treatment with methemoglobin-inducing agents since they are more susceptible to drug-induced methemoglobinemia.
- Signs and symptoms of methemoglobinemia may be delayed some hours after exposure. Initial signs and symptoms of methemoglobinemia include slate grey cyanosis seen in buccal mucus membranes, lips and nail beds. In severe cases, symptoms may include central cyanosis, headache, lethargy, dizziness, fatigue, syncope, dyspnea, CNS depression, seizures, dysrhythmia and shock. Methemoglobinemia should be considered if central cyanosis unresponsive to oxygen therapy occurs, especially if methemoglobin-inducing agents have been used. Calculated oxygen saturation and pulse oximetry are inaccurate in the setting of methemoglobinemia. The diagnosis can be confirmed by an elevated methemoglobin level of at least 10% is present. The development of methemoglobinemia is dose-related.
- Management of methemoglobinemia: If methemoglobinemia does not respond to administration of oxygen, clinically significant symptoms of methemoglobinemia should be treated with administration of a slow intravenous injection (over 5 minutes) of methylene blue at a dosage of 1-2 mg/kg body weight.
Anaphylaxis and Allergic-Type Reactions
- Articadent contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
Adverse Reactions
Clinical Trials Experience
- Reactions to articaine are characteristic of those associated with other amide-type local anesthetics. Adverse reactions to this group of drugs may also result from excessive plasma levels (which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation), injection technique, volume of injection, or hypersensitivity or they may be idiosyncratic.
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The reported adverse reactions are derived from clinical trials in the United States and the United Kingdom. Table 2 displays the adverse reactions reported in clinical trials where 882 individuals were exposed to Articadent containing epinephrine 1:100,000. Table 3 displays the adverse reactions reported in clinical trials where 182 individuals were exposed to Articadent containing epinephrine 1:100,000 and 179 individuals were exposed to Articadent containing epinephrine 1:200,000.
- Adverse reactions observed in at least 1% of patients:
Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of Articadent. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Persistent paresthesia of the lips, tongue, and oral tissues have been reported with use of articaine hydrochloride, with slow, incomplete, or no recovery. These postmarketing events have been reported chiefly following nerve blocks in the mandible and have involved the trigeminal nerve and its branches.
- Hypoesthesia has been reported with use of articaine, especially in pediatric age groups, which is usually reversible. Prolonged numbness can result in soft tissue injuries such as that of the lips and tongue in these age groups.
- Ischemic injury and necrosis have been described following use of articaine with epinephrine and have been postulated to be due to vascular spasm of terminal arterial branches.
- Paralysis of ocular muscles has been reported, especially after posterior, superior alveolar injections of articaine during dental anesthesia. Symptoms include diplopia, mydriasis, ptosis and difficulty in abduction of the affected eye. These symptoms have been described as developing immediately after injection of the anesthetic solution and persisting one minute to several hours, with generally complete recovery.
Drug Interactions
- The administration of local anesthetic solutions containing epinephrine to patients receiving monoamine oxidase inhibitors, nonselective beta-andregernic antagonists or tricyclic antidepressants may produce severe, prolonged hypertension. Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine. Concurrent use of these agents should be avoided; however, in situations when concurrent therapy is necessary, careful patient monitoring is essential
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies in pregnant women with Articadent. Articaine hydrochloride and epinephrine (1:100,000) has been shown to increase fetal deaths and skeletal variations in rabbits when given in doses approximately 4 times the maximum recommended human dose (MRHD). Articadent should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- In embryo-fetal toxicity studies in rabbits, 80 mg/kg, subcutaneously (approximately 4 times the MRHD based on body surface area) caused fetal death and increased fetal skeletal variations, but these effects may be attributable to the severe maternal toxicity, including seizures, observed at this dose. In contrast, no embryo-fetal toxicities were observed when articaine and epinephrine (1:100,000) was administered subcutaneously throughout organogenesis at doses up to 40 mg/kg in rabbits and 80 mg/kg in rats (approximately 2 times the MRHD based on body surface area).
- In pre- and postnatal developmental studies subcutaneous administration of articaine hydrochloride to pregnant rats throughout gestation and lactation, at a dose of 80 mg/kg (approximately 2 times the MRHD based on body surface area) increased the number of stillbirths and adversely affected passive avoidance, a measure of learning, in pups. This dose also produced severe maternal toxicity in some animals. A dose of 40 mg/kg (approximately equal to the MRHD on a mg/m2 basis) did not produce these effects. A similar study using articaine and epinephrine (1:100,000) rather than articaine hydrochloride alone produced maternal toxicity, but no effects on offspring.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Articaine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Articaine during labor and delivery.
Nursing Mothers
- It is not known whether Articadent is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Articadent is administered to a nursing woman. When using Articadent, nursing mothers may choose to pump and discard breast milk for approximately 4 hours (based on plasma half-life) following an injection of Articadent (to minimize infant ingestion) and then resume breastfeeding.
Pediatric Use
- Safety and effectiveness of Articadent in pediatric patients below the age of 4 years have not been established. Safety of doses greater than 7 mg/kg (0.175 mL/kg) in pediatric patients has not been established. Safety and effectiveness was established in clinical trials with 61 pediatric patients between the ages of 4 and 16 years administered articaine hydrochloride 4% with epinephrine 1:100,000 injections. Fifty-one of these patients received doses from 0.76 mg/kg to 5.65 mg/kg (0.9 mL to 5.1 mL) for simple dental procedures and 10 patients received doses between 0.37 mg/kg and 7.48 mg/kg (0.7 mL to 3.9 mL) for complex dental procedures. Approximately 13% of these pediatric patients required additional injections of anesthetic for complete anesthesia. Dosages in pediatric patients should be reduced, commensurate with age, body weight, and physical condition
Geriatic Use
- In clinical trials, 54 patients between the ages of 65 and 75 years, and 11 patients 75 years and over received Articadent containing epinephrine 1:100,000. Among all patients between 65 and 75 years, doses from 0.43 mg/kg to 4.76 mg/kg (0.9 to 11.9 mL) were administered to 35 patients for simple procedures and doses from 1.05 mg/kg to 4.27 mg/kg (1.3 to 6.8 mL) were administered to 19 patients for complex procedures. Among the 11 patients ≥ 75 years old, doses from 0.78 mg/kg to 4.76 mg/kg (1.3 to 11.9 mL) were administered to 7 patients for simple procedures and doses of 1.12 mg/kg to 2.17 mg/kg (1.3 to 5.1 mL) were administered to 4 patients for complex procedures.
- Approximately 6% of patients between the ages of 65 and 75 years and none of the 11 patients 75 years of age or older required additional injections of anesthetic for complete anesthesia compared with 11% of patients between 17 and 65 years old who required additional injections.
- No overall differences in safety or effectiveness were observed between elderly subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Articaine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Articaine with respect to specific racial populations.
Renal Impairment
- No studies have been performed with articaine hydrochloride 4% with epinephrine 1:200,000 injection or articaine hydrochloride 4% with epinephrine 1:100,000 injection in patients with renal or hepatic dysfunction
Hepatic Impairment
- No studies have been performed with articaine hydrochloride 4% with epinephrine 1:200,000 injection or articaine hydrochloride 4% with epinephrine 1:100,000 injection in patients with renal or hepatic dysfunction
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Articaine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Articaine in patients who are immunocompromised.
Administration and Monitoring
Administration
- submucosal infiltration or nerve block.
Monitoring
There is limited information regarding Monitoring of Articaine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Articaine in the drug label.
Overdosage
- Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local anesthetic solution.
- The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient's state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
- The first step in the management of convulsions, as well as hypo-ventilation, consists of immediate attention to the maintenance of a patient airway and assisted or controlled ventilation as needed. The adequacy of the circulation should be assessed. Should convulsions persist despite adequate respiratory support, treatment with appropriate anticonvulsant therapy is indicated. The practitioner should be familiar with the use of anticonvulsant drugs, prior to the use of local anesthetics. Supportive treatment of circulatory depression may require administration of intravenous fluids and, when appropriate, a vasopressor.
- If not treated immediately, both convulsions and cardiovascular depression can result in hypoxia, acidosis, bradycardia, arrhythmias and/or cardiac arrest. If cardiac arrest should occur, standard cardiopulmonary resuscitative measures should be instituted.
- For additional information about overdose treatment, call a poison control center
Pharmacology
Mechanism of Action
- Articaine HCl is an amide local anesthetic. Local anesthetics block the generation and conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of the affected nerve fibers. Epinephrine is a vasoconstrictor added to articaine HCl to slow absorption into the general circulation and thus prolong maintenance of an active tissue concentration.
Structure
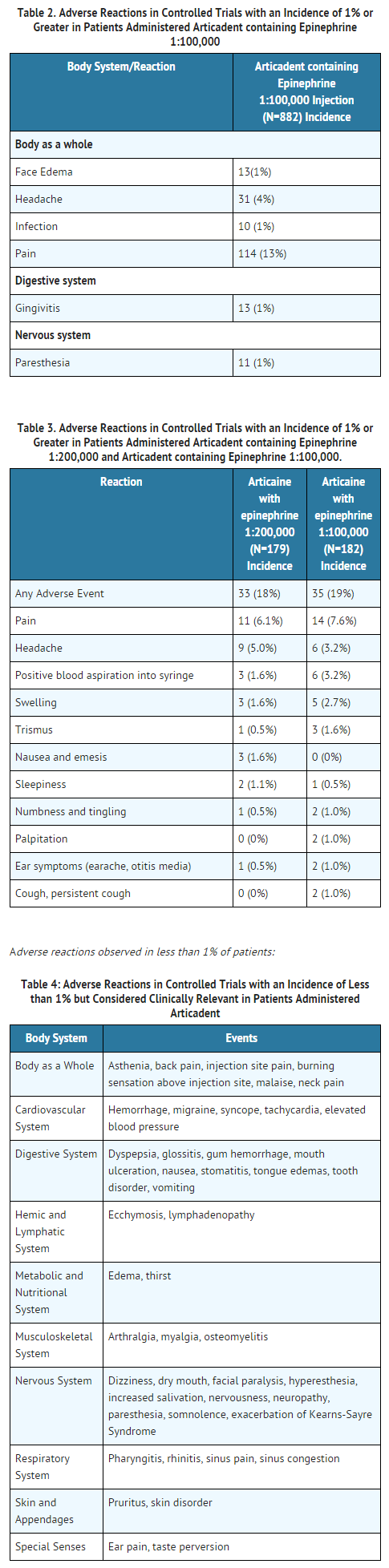
- Articadent injection is a sterile, aqueous solution that contains articaine HCl 4% (40 mg/mL) with epinephrine bitartrate in an epinephrine 1:200,000 or epinephrine 1:100,000 strength. Articaine HCl is an amino amide local anesthetic, chemically designated as 4-methyl-3-[2-(propylamino)-propionamido]-2-thiophene-carboxylic acid, methyl ester hydrochloride and is a racemic mixture. Articaine HCl has a molecular weight of 320.84 and the following structural formula:
- Articaine HCl has a partition coefficient in n-octanol/Soerensen buffer (pH: 7.35) of 17 and a pKa of 7.8.
- Epinephrine bitartrate, (-)-1-(3,4-dihydroxyphenyl)-2-methylamino-ethanol (+) tartrate (1:1) salt, is a vasoconstrictor that is added to articaine HCl in a concentration of 1:200,000 or 1:100,000 (expressed as free base). It has a molecular weight of 333.3 and the following structural formula:
Pharmacodynamics
- Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
- The onset of anesthesia has been shown to be within 1 to 9 minutes of injection of ARTICADENT. Complete anesthesia lasts approximately 1 hour for infiltrations and up to approximately 2 hours for nerve block.
- Administration of Articadent results in a 3- to 5-fold increase in plasma epinephrine concentrations compared to baseline; however, in healthy adults it does not appear to be associated with marked increases in blood pressure or heart rate, except in the case of accidental intravascular injection
Pharmacokinetics
Absorption
- Following dental injection by the submucosal route of an articaine solution containing epinephrine1:200,000, articaine reaches peak blood concentration about 25 minutes after a single dose injection and 48 minutes after three doses. Peak plasma levels of articaine achieved after 68 and 204 mg doses are 385 and 900 ng/mL, respectively. Following intraoral administration of a near maximum dose of 476 mg, articaine reaches peak blood concentrations of 2037 and 2145 ng/mL for articaine solution containing epinephrine 1:100,000 and 1:200,000, respectively, approximately 22 minutes post-dose.
Distribution
- Approximately 60 to 80% of articaine HCl is bound to human serum albumin and γ-globulins at 37°C in vitro.
Metabolism
- Articaine HCl is metabolized by plasma carboxyesterase to its primary metabolite, articainic acid, which is inactive. In vitro studies show that the human liver microsome P450 isoenzyme system metabolizes approximately 5% to 10% of available articaine with nearly quantitative conversion to articainic acid.
Excretion
- At the dose of 476 mg of articaine, the elimination half-life was 43.8 minutes and 44.4 minutes for articaine solution with epinephrine 1:100,000 and 1:200,000, respectively. Articaine is excreted primarily through urine with 53% to 57% of the administered dose eliminated in the first 24 hours following submucosal administration. Articainic acid is the primary metabolite in urine. A minor metabolite, articainic acid glucuronide, is also excreted in urine. Articaine constitutes only 2% of the total dose excreted in urine.
Special Populations
- No studies have been performed to evaluate the pharmacokinetics of Articadent injection in pediatric subjects. There is insufficient information to determine whether the pharmacokinetics of ARTICADENT differs by race.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Studies to evaluate the carcinogenic potential of articaine HCI in animals have not been conducted. Five standard mutagenicity tests, including three in vitro tests (the nonmammalian Ames test, the mammalian Chinese hamster ovary chromosomal aberration test and a mammalian gene mutation test with articaine HCl) and two in vivo mouse micronucleous tests (one with articaine and epinephrine 1:100,000 and one with articaine HCl alone) showed no mutagenic effects.
- No effects on male or female fertility were observed in rats for articaine and epinephrine 1:100,000 administered subcutaneously in doses up to 80 mg/kg/day (approximately 2 times the MRHD based on body surface area).
Clinical Studies
- Three randomized, double-blind, active-controlled studies were designed to evaluate the effectiveness of Articadent containing epinephrine 1:100,000 as a dental anesthetic. Patients ranging in age from 4 years to over 65 years old underwent simple dental procedures such as single uncomplicated extractions, routine operative procedures, single apical resections, and single crown procedures, or complex dental procedures such as multiple extractions, multiple crowns and/or bridge procedures, multiple apical resections, alveolectomies, muco-gingival operations, and other surgical procedures on the bone. Articadent containing epinephrine 1:100,000 was administered as submucosal infiltration and/or nerve block. Efficacy was measured immediately following the procedure by having the patient and investigator rate the patient's procedural pain using a 10 cm visual analog scale (VAS), in which a score of zero represented no pain and a score of 10 represented the worst pain imaginable. Mean patient and investigator VAS pain scores were 0.3 cm-0.4 cm for simple procedures and 0.5 cm-0.6 cm for complex procedures.
- Four randomized, double-blind, active-controlled studies were performed comparing Articadent containing epinephrine 1:100,000 versus Articadent containing epinephrine 1:200,000. The first two studies used electric pulp testers (EPT) to evaluate the success rate (maximum EPT value within 10 minutes), onset, and duration of Articadent containing epinephrine 1:100,000 versus Articadent containing epinephrine 1:200,000 and articaine solution without epinephrine in healthy adults between 18 and 65 years old. Results indicated that the anesthetic characteristics of the 1:100,000 and 1:200,000 formulations were not significantly different.
- A third study compared the difference in visualization of the surgical field after administration of Articadent containing epinephrine 1:100,000 versus Articadent containing epinephrine1:200,000 during bilateral maxillary periodontal surgeries in patients ranging from 21 to 65 years old. Articadent containing epinephrine1:100,000 provided better visualization of the surgical field and less blood loss during the procedures. In a fourth study, designed to assess and compare cardiovascular safety, when the maximum dose of each formulation was administered, no clinically relevant differences in blood pressure or heart rate between formulations were observed.
How Supplied
- Articadent (articaine HCl with epinephrine) Injection is available in 1.7 mL single use glass cartridges, packaged in boxes of 50 cartridges in the following two strengths:
- Articadent containing articaine HCl 4% (40 mg/mL) and epinephrine 1:200,000 (as epinephrine bitartrate 0.009 mg/mL) (NDC 66312-602-16)
- Articadent containing articaine HCl 4% (40 mg/mL) and epinephrine 1:100,000 (as epinephrine bitartrate 0.018 mg/mL) (NDC 66312-601-16)
Storage
- Store at controlled room temperature 25°C (77°F) with brief excursions permitted between 15° and 30°C (59°F - 86°F) [see USP Controlled Room Temperature]. Protect from light. Do Not Freeze.
- For chemical disinfection of the carpule, either isopropyl alcohol (91%) or ethyl alcohol (70%) is recommended. Many commercially available brands of isopropyl (rubbing) alcohol, as well as solutions of ethyl alcohol not of U.S.P. grade, contain denaturants that are injurious to rubber and therefore are not to be used.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Images
Drug Images
{{#ask: Page Name::Articaine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 1.7 ML CARTRIDGE CARTON (EPINEPHRINE 1:100,000)
NDC 66312-601-16
DENTSPLY PHARMACEUTICAL
Articadent® DENTAL (articaine HCl and epinephrine) Injection
Articaine hydrochloride 4% and epinephrine 1:100,000
Intraoral Submucosal Injection Contains sodium metabisulfite Store at 25°C (77°F) DO NOT PERMIT TO FREEZE. Rx only 50 Cartridges, 1.7 mL each
COLOR CODED
STERILE AQUEOUS SOLUTION FOR INJECTION
DENTSPLY Reorder #: 51116
PRINCIPAL DISPLAY PANEL - 1.7 ML CARTRIDGE CARTON (EPINEPHRINE 1:200,000)
NDC 66312-602-16
DENTSPLY PHARMACEUTICAL
Articadent® DENTAL (articaine HCl and epinephrine) Injection
Articaine hydrochloride 4% and epinephrine 1:200,000
Intraoral Submucosal Injection Contains sodium metabisulfite Store at 25°C (77°F) DO NOT PERMIT TO FREEZE. Rx only 50 Cartridges, 1.7 mL each
COLOR CODED
STERILE AQUEOUS SOLUTION FOR INJECTION
DENTSPLY Reorder #: 52216
Ingredients and Appearance
{{#ask: Label Page::Articaine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Loss of Sensation and Muscle Function:
- Inform patients in advance of the possibility of temporary loss of sensation and muscle function following infiltration and nerve block injections.
- Instruct patients not to eat or drink until normal sensation returns.
Precautions with Alcohol
- Alcohol-Articaine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ARTICADENT ®[2]
Look-Alike Drug Names
There is limited information regarding Articaine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Oertel R, Ebert U, Rahn R, Kirch W. Clinical pharmacokinetics of articaine. Clin Pharmacokinet. 1997 Dec;33(6):421
- ↑ "Articaine hydrochloride and epinephrine".