Aromatic L-amino acid decarboxylase
| aromatic-L-amino-acid decarboxylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 4.1.1.28 | ||||||||
| CAS number | 9042-64-2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| DOPA decarboxylase (aromatic L-amino acid decarboxylase) | |
|---|---|
| Identifiers | |
| Symbol | DDC |
| Entrez | 1644 |
| HUGO | 2719 |
| OMIM | 107930 |
| RefSeq | NM_000790 |
| UniProt | P20711 |
| Other data | |
| EC number | 4.1.1.28 |
| Locus | Chr. 7 p11 |
Aromatic L-amino acid decarboxylase (AADC or AAAD), also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme (EC 4.1.1.28).
Reactions
AADC catalyzes several different decarboxylation reactions:[2]
- L-DOPA to dopamine - a neurotransmitter
- L-phenylalanine to phenethylamine - a trace amine neuromodulator
- L-tyrosine to tyramine - a trace amine neuromodulator
- L-histidine to histamine - a neurotransmitter
- L-tryptophan to tryptamine - a trace amine neuromodulator
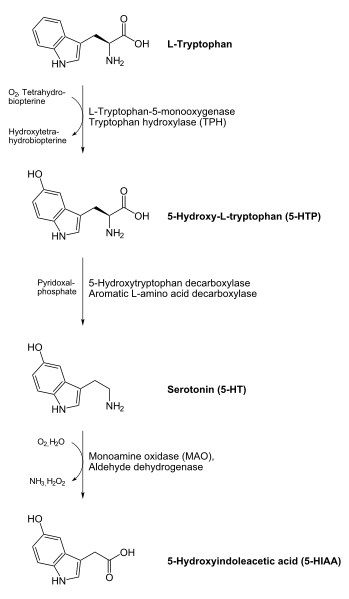
- 5-HTP to serotonin (5-hydroxytryptamine) - a neurotransmitter
The enzyme uses pyridoxal phosphate, the active form of vitamin B6, as a cofactor.
In humans, catecholamines and phenethylaminergic trace amines are derived from the amino acid L-phenylalanine.
|
As a rate-limiting step
In normal dopamine and serotonin (5-HT) neurotransmitter synthesis, AADC is not the rate-limiting step in either reaction. However, AADC becomes the rate-limiting step of dopamine synthesis in patients treated with L-DOPA (such as in Parkinson's disease), and the rate-limiting step of serotonin synthesis in people treated with 5-HTP (such as in mild depression or dysthymia). AADC is inhibited by carbidopa outside of the blood brain barrier to inhibit the premature conversion of L-DOPA to dopamine in the treatment of Parkinson's.
In humans, AADC is also the rate-limiting enzyme in the formation of trace amines. Deficiency of AADC is associated with various symptoms as severe developmental delay, oculogyric crises and autonomic dysfunction. The molecular and clinical spectrum of AAAC deficiency is heterogeneous. The first case of AADC deficiency was described in twin brothers 1990. Patients can be treated with dopamine agonists, MAO inhibitors, and pyridoxine (vitamin B6).[6] Clinical phenotype and response to treatment is variable and the long-term and functional outcome is unknown. To provide a basis for improving the understanding of the epidemiology, genotype/phenotype correlation and outcome of these diseases their impact on the quality of life of patients, and for evaluating diagnostic and therapeutic strategies a patient registry was established by the noncommercial International Working Group on Neurotransmitter Related Disorders (iNTD).[7]
Genetics
The gene encoding the enzyme is referred to as DDC and located on chromosome 7 in humans.[8] Single nucleotide polymorphisms and other gene variations have been investigated in relation to neuropsychiatric disorders, e.g., a one-base pair deletion at –601 and a four-base pair deletion at 722–725 in exon 1 in relation to bipolar disorder[9] and autism. No direct correlation between gene variation and autism was found.[10]
See also
- Aromatic L-amino acid decarboxylase inhibitor, a class of anti-Parkinson drugs
- Aromatic amino acids
- Histidine decarboxylase
References
- ↑ PDB: 1JS3; Burkhard P, Dominici P, Borri-Voltattorni C, Jansonius JN, Malashkevich VN (Nov 2001). "Structural insight into Parkinson's disease treatment from drug-inhibited DOPA decarboxylase". Nature Structural Biology. 8 (11): 963–7. doi:10.1038/nsb1101-963. PMID 11685243.
- ↑ "AADC". Human Metabolome database. Retrieved 17 February 2015.
- ↑ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ↑ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ↑ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". Eur. J. Pharmacol. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ↑ Pons R, Ford B, Chiriboga CA, Clayton PT, Hinton V, Hyland K, Sharma R, De Vivo DC (Apr 2004). "Aromatic L-amino acid decarboxylase deficiency: clinical features, treatment, and prognosis". Neurology. 62 (7): 1058–65. doi:10.1212/WNL.62.7.1058. PMID 15079002.
- ↑ "Patient registry".
- ↑ Scherer LJ, McPherson JD, Wasmuth JJ, Marsh JL (Jun 1992). "Human dopa decarboxylase: localization to human chromosome 7p11 and characterization of hepatic cDNAs". Genomics. 13 (2): 469–71. doi:10.1016/0888-7543(92)90275-W. PMID 1612608.
- ↑ Børglum AD, Bruun TG, Kjeldsen TE, Ewald H, Mors O, Kirov G, Russ C, Freeman B, Collier DA, Kruse TA (Nov 1999). "Two novel variants in the DOPA decarboxylase gene: association with bipolar affective disorder". Molecular Psychiatry. 4 (6): 545–51. doi:10.1038/sj.mp.4000559. PMID 10578236.
- ↑ Lauritsen MB, Børglum AD, Betancur C, Philippe A, Kruse TA, Leboyer M, Ewald H (May 2002). "Investigation of two variants in the DOPA decarboxylase gene in patients with autism". American Journal of Medical Genetics. 114 (4): 466–70. doi:10.1002/ajmg.10379. PMID 11992572.
External links
- Aromatic-L-Amino-Acid+Decarboxylases at the US National Library of Medicine Medical Subject Headings (MeSH)