Antithrombin
| Serpin peptidase inhibitor, clade C (antithrombin), member 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbols | SERPINC1 ; AT3; ATIII; MGC22579 | ||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 20139 | ||||||||||
| |||||||||||
| RNA expression pattern | |||||||||||
 | |||||||||||
| More reference expression data | |||||||||||
| Orthologs | |||||||||||
| Template:GNF Ortholog box | |||||||||||
| Species | Human | Mouse | |||||||||
| Entrez | n/a | n/a | |||||||||
| Ensembl | n/a | n/a | |||||||||
| UniProt | n/a | n/a | |||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||
| RefSeq (protein) | n/a | n/a | |||||||||
| Location (UCSC) | n/a | n/a | |||||||||
| PubMed search | n/a | n/a | |||||||||
|
WikiDoc Resources for Antithrombin |
|
Articles |
|---|
|
Most recent articles on Antithrombin Most cited articles on Antithrombin |
|
Media |
|
Powerpoint slides on Antithrombin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Antithrombin at Clinical Trials.gov Clinical Trials on Antithrombin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Antithrombin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Antithrombin Discussion groups on Antithrombin Patient Handouts on Antithrombin Directions to Hospitals Treating Antithrombin Risk calculators and risk factors for Antithrombin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Antithrombin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editors-In-Chief: C. Michael Gibson, M.S., M.D. Duke Clinical Research Institute [1]; John Alexander, M.D. [2], Duke Clinical Research Institute
Overview
Antithrombin (AT) is a small protein molecule that inactivates several enzymes of the coagulation system. It is a glycoprotein produced by the liver and consists of 432 amino acids. It contains three disulfide bonds and a total of four possible glycosylation sites. α-antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin.[1]
Antithrombin Nomenclature
Antithrombin is also termed Antithrombin III (AT III). The designations Antithrombin I through to Antithrombin IV originate in early studies carried out in the 1950s by Seegers, Johnson and Fell.[2]
Antithrombin I (AT I) refers to the absorption of thrombin onto fibrin after thrombin has activated fibrinogen. Antithrombin II (AT II) refers to a cofactor in plasma, which together with heparin interferes with the interaction of thrombin and fibrinogen. Antithrombin III (AT III) refers to a substance in plasma which inactivates thrombin, whose activity is independent of heparin. Antithrombin IV (AT IV) refers to an antithrombin which becomes activated during and shortly after blood coagulation.[3] Only AT III and possibly AT I are medically significant. AT III is generally referred to solely as "Antithrombin" and it is Antithrombin III that is discussed in this article.
Structure
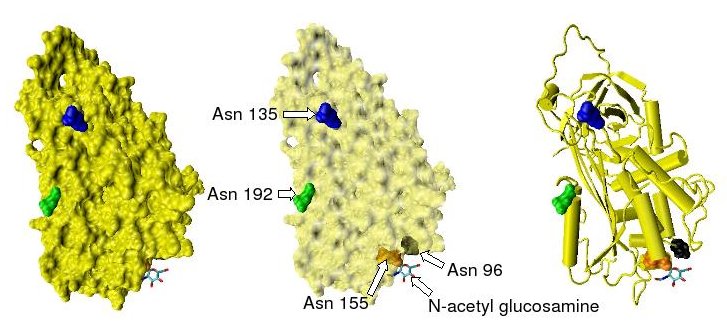
Antithrombin has a half life in blood plasma of around 3 days.[4] The normal antithrombin concentration in human blood plasma is high at approximately 0.12 mg/ml, which is equivalent to a molar concentration of 2.3 μM.[5] Antithrombin has been isolated from the plasma of a large number of species additional to humans.[6] As deduced from protein and cDNA sequencing, cow, sheep, rabbit and mouse antithrombins are all 433 amino acids in length, which is one amino acid longer than human antithrombin III. The extra amino acid is thought to occur at amino acid position 6. Cow, sheep, rabbit, mouse and human antithrombins share between 84 and 89% amino acid sequence identity.[7] They all have four potential N-glycosylation sites. These occur at asparagine (Asn) amino acid numbers 96, 135, 155 and 192 in humans and at similar amino acid numbers in other species. All these sites are occupied by covalently attached oligosaccharide side chains in the predominant form of human antithrombin, α-antithrombin, resulting in a molecular weight for this form of antithrombin of 58,200.[1] The potential glycosylation site at asparagine 135 is not occupied in a minor form of antithrombin, β-antithrombin (see Figure 1).[8]
Recombinant antithrombins with properties similar to those of normal human antithrombin have been produced using baculovirus-infected insect cells and mammalian cell lines grown in cell culture.[9][10][11][12] These recombinant antithrombins generally have different glycosylation patterns to normal antithrombin and are typically used in antithrombin structural studies. For this reason many of the antithrombin structures stored in the protein data bank and presented in this article show variable glycosylation patterns.
Function

Antithrombin is a serpin (serine protease inhibitor) and is thus similar in structure to most other plasma protease inhibitors, such as alpha 1-antichymotrypsin, alpha 2-antiplasmin and heparin cofactor II.
The physiological target proteases of antithrombin are those of the contact activation pathway (formerly known as the intrinsic pathway), namely the activated forms of factor X (Xa), factor IX (IXa), factor XI (XIa), factor XII (XIIa) and factor II (thrombin) (IIa) and also the activated form of factor VII (VIIa) from the tissue factor pathway (formerly known as the extrinsic pathway).[15] The inhibitor also inactivates kallikrein and plasmin, also involved in blood coagulation. However it inactivates certain other serine proteases that are not involved in coagulation such as trypsin and the C1s subunit of the enzyme C1 involved in the classical complement pathway.[7][16]
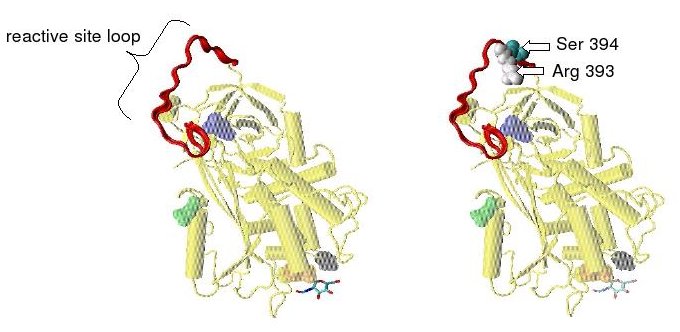
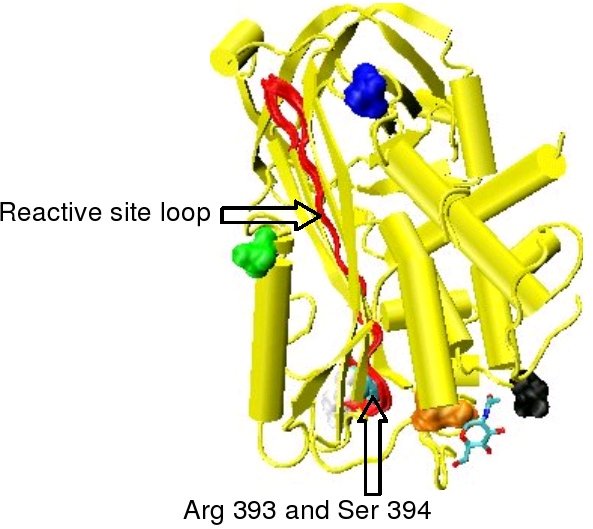
Protease inactivation results as a consequence of the trapping the protease in an equimolar complex with antithrombin in which the active site of the protease enzyme is inaccessible to its usual substrate.[7] The formation of an antithrombin-protease complex involves an interaction between the protease and a specific reactive peptide bond within antithrombin. In human antithrombin this bond is between arginine (arg) 393 and serine (ser) 394 (see Figure 2 and Figure 3).[7]
It is thought the trapping of protease enzymes in inactive antithrombin-protease complexes results as a consequence of their attack of the reactive bond. Where the attack of a similar bond within their normal substrate results in its rapid proteolytic cleavage, on initiating an attack on the antithrombin reactive bond the antithrombin inhibitor is activated to trap the enzyme at an intermediate stage during the proteolytic process. Given time thrombin is able to cleave the reactive bond within antithrombin and an inactive antithrombin-thrombin complex will dissociate, however the time it takes for this to occur may be greater than 3 days.[17] However bonds P3-P4 and P1'-P2' can be rapidly cleaved by neutrophil elastase and thermolysin respectively, resulting in inactive antithrombins no longer able inhibit thrombin activity.[18]
The rate of antithrombin's inhibition of protease activity is greatly enhanced by its additional binding to heparin as is its inactivation by neutrophil elastase.[18]
Antithrombin and Heparin
Antithrombin inactivates its physiological target enzymes, thrombin, factor Xa and factor IXa with rate constants of 7 - 11 x 10-3, 2.5 x 10-3 M-1 s-1 and 1 x 10 M-1 s-1 respectively.[1][19] The rate of antithrombin-thrombin inactivation increases to 1.5 - 4 x 107 M-1 s-1 in the presence of heparin, i.e the reaction is accelerated 2000-4000 fold.[20][21][22][23] Factor Xa inhibition is accelerated by only 500 to 1000 fold in the presence of heparin and the maximal rate constant is 10 fold lower than that of thrombin inhibition.[20][23] The rate enhancement of antithrombin-Factor IXa inhibition shows an approximate 1 million fold enhancement in the presence of heparin and physiological levels of calcium.[19]
AT-III binds to a specific pentasaccharide sulfation sequence contained within the heparin polymer.
GlcNAc/NS(6S)-GlcA-GlcNS(3S,6S)-IdoA(2S)-GlcNS(6S)
Upon binding to this pentasaccharide sequence, inhibition of protease activity is increased by heparin as a result of two distinct mechanisms.[24] In one mechanism heparin stimulation of Factor IXa and Xa inhibition depends on a conformational change within antithrombin involving the reactive site loop and is thus allosteric.[25] In another mechanism stimulation of thrombin inhibition depends on the formation of a ternary complex between AT-III, thrombin, and heparin.[25]
Allosteric Activation

Increased Factor IXa and Xa inhibition requires the minimal heparin pentasaccharide sequence. The conformational changes that occur within antithrombin in response to pentasaccharide binding are well documented.[13][26][27]
In the absence of heparin, amino acids P14 and P15 (see Figure 3) from the reactive site loop are embedded within the main body of the protein (specifically the top of beta sheet A). This feature is in common with other serpins such as heparin cofactor II, alpha 1-antichymotrypsin and MENT.
The conformational change most relevant for Factor IXa and Xa inhibition involves the P14 and P15 amino acids within the N-terminal region of the reactive site loop (circled in Figure 4 model B). This region has been termed the hinge region. The conformational change within the hinge region in response to heparin binding results in the expulsion of P14 and P15 from the main body of the protein and it has been shown that by preventing this conformational change, increased Factor IXa and Xa inhibition does not occur.[25] It is thought that the increased flexibility given to the reactive site loop as a result of the hinge region conformational change is a key factor in influencing increased Factor IXa and Xa inhibition. It has been calculated that in the absence of the pentasaccharide only one in every 400 Antithrombin molecules (0.25%) is in an active conformation with the P14 and P15 amino acids expelled.[25]
Non-allosteric Activation

Increased thrombin inhibition requires the minimal heparin pentasaccharide plus at least an additional 13 monomeric units.[28] This is thought to be due to a requirement that antithrombin and thrombin must bind to the same heparin chain adjacent to each other. This can be seen in the series of models shown in Figure 5.
In the structures shown in Figure 5 the C-terminal portion (P' side) of the reactive site loop is in an extended conformation when compared with other un-activated or heparin activated antithrombin structures.[29] The P' region of antithrombin is unusually long relative to the P' region of other serpins and in un-activated or heparin activated antithrombin structures forms a tightly hydrogen bonded β-turn. P' elongation occurs through the breaking of all hydrogen bonds involved in the β-turn.[29]
The hinge region of antithrombin in the Figure 5 complex could not be modelled due to its conformational flexibility and amino acids P9-P14 are not seen in this structure. This conformational flexibility indicates an equilibrium may exist within the complex between a P14 P15 reactive site loop inserted antithrombin conformation and a P14 P15 reactive site loop expelled conformation. In support of this, analysis of the positioning of P15 Gly in the Figure 5 complex shows it to be inserted into beta sheet A.[29]
Effect of Glycosylation on Activity
α-antithrombin and β-antithrombin differ in their affinity for heparin.[30] The difference in dissociation constant between the two is threefold for the pentasaccharide shown in Figure 3 and over tenfold for full length heparin, with β-antithrombin having a higher affinity.[31] The higher affinity of β-antithrombin is thought to be due to the increased rate at which subsequent conformational changes occur within the protein upon initial heparin binding. For α-antithrombin, the additional glycosylation at Asn-135 is not thought to interfere with initial heparin binding, but however to inhibit any resulting conformational changes.[30]
Even though it is only present at 5-10% the levels of α-antithrombin, due to its increased heparin affinity, it is thought that β-antithrombin is more important than α-antithrombin in controlling thrombogenic events resulting from tissue injury. Indeed, thrombin inhibition after injury to the aorta has been attributed solely to β-antithrombin.[32]
Cleaved and Latent Antithrombin
Cleavage at the reactive site results in entrapment of the thrombin protease, with movement of the cleaved reactive site loop together with the bound protease, such that the loop forms an extra sixth strand in the middle of beta sheet A. This movement of the reactive site loop can also be induced without cleavage, with the resulting crystallographic structure being identical to that of the physiologically latent conformation of plasminogen activator inhibitor-1 (PAI-1).[33] For this reason the conformation of antithrombin in which the reactive site loop is incorporated uncleaved into the main body of the protein is referred to as latent antithrombin. In contrast to PAI-1 the transition for antithrombin from a normal or native conformation to a latent conformation is irreversible.
Native antithrombin can be converted to latent antithrombin (L-antithrombin) by heating alone or heating in the presence of citrate.[34][35] However, without extreme heating and at 37°C (body temperature) 10% of all antithrombin circulating in the blood is converted to the L-antithrombin over a 24 hour period.[36][37] The structure of L-antithrombin is shown in Figure 6.
The 3-dimensional structure of native antithrombin was first determined in 1994.[26][27] Unexpectedly the protein crystallized as a heterodimer composed of one molecule of native antithrombin and one molecule of latent antithrombin. Latent antithrombin on formation immediately links to a molecule of native antithrombin to form the heterodimer, and it is not until the concentration of latent antithrombin exceeds 50% of the total antithrombin that it can be detected analytically.[37] Not only is the latent form of antithrombin inactive, but its dimerisation with an otherwise active native antithrombin molecule also results in the native molecules inactivation. The physiological impact of the loss of antithrombin activity either through latent antithrombin formation or subsequent dimer formation, is exacerbated by the preference for dimerisation to occur between heparin activated β-antithrombin and latent antithrombin as opposed to α-antithrombin.[37]
Role in Disease
Evidence for the important role antithrombin plays in regulating normal blood coagulation is demonstrated by the correlation between inherited or acquired antithrombin deficiencies and an increased risk of any affected individual developing thrombotic disease.[38] Antithrombin deficiency generally comes to light when a patient suffers recurrent venous thrombosis and pulmonary embolism.
Acquired Antithrombin Deficiency
Acquired antithrombin deficiency may result from a range of disorders such as liver dysfunction (coagulopathy), sepsis, premature birth, kidney disease with protein loss in the urine in patients with nephrotic syndrome, or as a result of interventions such as major surgery or cardiopulmonary bypass.[39]
Inherited Antithrombin Deficiency
Inherited deficiencies in blood antithrombin activity may be due to a low circulating level of structurally and functionally normal antithrombin. In this case typically antithrombin levels and therefore activity may be reduced by 50% compared to normal antithrombin levels.[40] This type of deficiency is classified as type I antithrombin deficiency.
Inherited deficiencies may also be the result of a structurally and functionally abnormal antithrombin protein circulating in the blood. In this case levels of antithrombin may be normal but the activity produced by this protein again may be reduced by 50% when compared to normal antithrombin activity levels.[40] This type of deficiency is classified as type II antithrombin deficiency.
Both type I and type II antithrombin deficiency have been shown to be the result of any one of a number of frameshift mutations, missense mutations or nonsense mutations in the gene that encodes antithrombin.[40][41][42]
Antithrombin Deficiency Patient Advocacy
Patients and families with antithrombin deficiency are organized in the non-profit patient organization NATT (National Alliance for Thrombosis and Thrombophilia). A detailed medical information antithrombin deficiency brochure is available through NATT.
References
- ↑ 1.0 1.1 1.2 Bjork, I (1997). Antithrombin, A bloody important serpin (in Chemistry and Biology of Serpins). Plenum Press. pp. 17–33. ISBN 0-306-45698-2. Unknown parameter
|coauthors=ignored (help) - ↑ Seegers WH, Johnson JF, Fell C. (1954). "An antithrombin reaction to prothrombin activation". Am. J. Physiol. 176 (1): 97–103. PMID 13124503.
- ↑ Yin ET, Wessler S. and Stoll PJ. (1971). "Identity of plasma-activated factor X inhibitor with antithrombin 3 and heparin cofactor". J. Biol. Chem. 246 (11): 3712–3719. PMID 4102937.
- ↑ Collen DJ, Schetz F.; et al. (1977). "Metabolism of antithrombin III (heparin cofactor) in man: Effects of venous thrombosis of heparin administration". Eur. J. Clin. Invest. 7: 27–35. doi:10.1111/j.1365-2362.1977.tb01566.x. PMID 65284.
- ↑ Conrad J, Brosstad M.; et al. (1983). "Molar antithrombin concentration in normal human plasma". Haemostasis. 13: 363–368. PMID 6667903.
- ↑ Jordan RE. (1983). "Antithrombin in vertebrate species: Conservation of the heparin-dependent anticoagulant mechanism". Arch. Biochem. Biophys. 227: 587–595. doi:10.1016/0003-9861(83)90488-5. PMID 6607710.
- ↑ 7.0 7.1 7.2 7.3 Olson ST, Bjork I. (1994). "Regulation of thrombin activity by antithrombin and heparin". Sem. Thromb. Hemost. 20 (4): 373–409. PMID 7899869.
- ↑ Brennan SO, George PM, Jordan, RE. (1987). "Physiological variant of antithrombin-III lacks carbohydrate side chain at Asn 135". FEBS Lett. 219: 431–436. doi:10.1016/0014-5793(87)80266-1. PMID 3609301.
- ↑ Stephens AW, Siddiqui A. and Hirs CH. (1987). "Expression of functionally active human antithrombin III". Proc. Natl. Acad. Sci. USA. 84 (11): 3886–3890. doi:10.1073/pnas.84.11.3886. PMID 3473488.
- ↑ Zettlmeissl G, Conradt HS.; et al. (1989). "Characterization of recombinant human antithrombin III synthesized in Chinese hamster ovary cells". J. Biol. Chem. 264 (35): 21153–21159. PMID 2592368.
- ↑ Gillespie LS, Hillesland KK and Knauer DJ. (1991). "Expression of biologically active human antithrombin III by recombinant baculovirus in Spodoptera frugiperda cells". J. Biol. Chem. 266 (6): 3995–4001. PMID 1995647.
- ↑ Ersdal-Badju E, Lu A.; et al. (1995). "Elimination of glycosylation heterogeneity affecting heparin affinity of recombinant human antithrombin III by expression of a beta-like variant in baculovirus-infected insect cells". Biochem. J. 310: 323–330. PMID 7646463.
- ↑ 13.0 13.1 Whisstock JC, Pike RN.; et al. (2000). "Conformational changes in serpins: II. The mechanism of activation of antithrombin by heparin". J. Mol. Biol. 301 (5): 1287–1305. doi:10.1006/jmbi.2000.3982. PMID 10966821.
- ↑ Schechter I and Berger A. (1967). "On the size of the active site in proteases. I. Papain". Biochem. Biophys. Res. Commun. 27 (2): 157–162. doi:10.1016/S0006-291X(67)80055-X. PMID 6035483.
- ↑ Persson E, Bak H and Olsen OH. (2001). "Substitution of valine for leucine 305 in factor VIIa increases the intrinsic enzymatic activity". J. Biol. Chem. 276 (31): 29195–29199. doi:10.1074/jbc.M102187200. PMID 11389142.
- ↑ Ogston D, Murray J. and Crawford GP. (1976). "Inhibition of the activated Cls subunit of the first component of complement by antithrombin III in the presence of heparin". Thromb. Res. 9 (3): 217–222. doi:10.1016/0049-3848(76)90210-3. PMID 982345.
- ↑ Danielsson A and Bjork, I (1980). "Slow, spontaneous dissociation of the antithrombin-thrombin complex produces a proteolytically modified form of the inhibitor". FEBS Lett. 119: 241–244. doi:10.1016/0014-5793(80)80262-6. PMID 7428936.
- ↑ 18.0 18.1 Chang WS, Wardell MR.; et al. (1996). "Probing serpin reactive-loop conformations by proteolytic cleavage". Biochem. J. 314 (2): 647–653. PMID 8670081.
- ↑ 19.0 19.1 Bedsted T, Swanson R.; et al. (2003). "Heparin and calcium ions dramatically enhance antithrombin reactivity with factor IXa by generating new interaction exosites". Biochemistry. 42 (27): 8143–8152. doi:10.1021/bi034363y. PMID 12846563.
- ↑ 20.0 20.1 Jordan RE, Oosta GM.; et al. (1980). "The kinetics of hemostatic enzyme-antithrombin interactions in the presence of low molecular weight heparin". J. Biol. Chem. 255 (21): 10081–10090. PMID 6448846.
- ↑ Griffith MJ. (1982). "Kinetics of the heparin-enhanced antithrombin III/thrombin reaction. Evidence for a template model for the mechanism of action of heparin". J. Biol. Chem. 257 (13): 7360–7365. PMID 7085630.
- ↑ Olson ST. and Björk I. (1991). "Predominant contribution of surface approximation to the mechanism of heparin acceleration of the antithrombin-thrombin reaction. Elucidation from salt concentration effects". J. Biol. Chem. 266 (10): 6353–6354. PMID 2007588.
- ↑ 23.0 23.1 Olson ST, Björk I.; et al. (1992). "Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement". J. Biol. Chem. 267 (18): 12528–12538. PMID 1618758.
- ↑ Johnson DJ, Langdown J; et al. (2006). "Crystal structure of monomeric native antithrombin reveals a novel reactive center loop conformation". J. Biol. Chem. 281 (46): 35478–35486. doi:10.1074/jbc.M607204200. PMID 16973611.
- ↑ 25.0 25.1 25.2 25.3 Langdown J, Johnson DJ.; et al. (2004). "Allosteric activation of antithrombin critically depends upon hinge region extension". J. Biol. Chem. 279 (45): 47288–47297. doi:10.1074/jbc.M408961200. PMID 15326167.
- ↑ 26.0 26.1 Schreuder HA, de Boer B.; et al. (1994). "The intact and cleaved human antithrombin III complex as a model for serpin-proteinase interactions". Nat. Struct. Biol. 1 (1): 48–54. doi:10.1038/nsb0194-48. PMID 7656006.
- ↑ 27.0 27.1 Carrell RW, Stein PE.; et al. (1994). "Biological implications of a 3 A structure of dimeric antithrombin". Structure. 2 (4): 257–270. doi:10.1016/S0969-2126(00)00028-9. PMID 8087553.
- ↑ Petitou M, Herault JP, Bernat A, Driguez PA; et al. (1999). "Synthesis of Thrombin inhibiting Heparin mimetics without side effects". Nature. 398: 417–422. PMID 10201371.
- ↑ 29.0 29.1 29.2 Li W, Johnson DJ.; et al. (2004). "Structure of the antithrombin-thrombin-heparin ternary complex reveals the antithrombotic mechanism of heparin". Nat. Struct. Mol. Biol. 11 (9): 857–862. doi:10.1038/nsmb811. PMID 15311269.
- ↑ 30.0 30.1 McCoy AJ, Pei XY.; et al. (2003). "Structure of beta-antithrombin and the effect of glycosylation on antithrombin's heparin affinity and activity". J. Mol. Biol. 326 (3): 823–833. doi:10.1016/S0022-2836(02)01382-7. PMID 12581643.
- ↑ Turk B, Brieditis I.; et al. (1997). "The oligosaccharide side chain on Asn-135 of alpha-antithrombin, absent in beta-antithrombin, decreases the heparin affinity of the inhibitor by affecting the heparin-induced conformational change". Biochemistry. 36 (22): 6682–6691. doi:10.1021/bi9702492. PMID 9184148.
- ↑ Frebelius S, Isaksson S. and Swedenborg J. (1996). "Thrombin inhibition by antithrombin III on the subendothelium is explained by the isoform AT beta". Arterioscler. Thromb. Vasc. Biol. 16 (10): 1292–1297. PMID 8857927.
- ↑ Mottonen J, Strand A.; et al. (1992). "Structural basis of latency in plasminogen activator inhibitor-1". Nature. 355 (6357): 270–273. doi:10.1038/355270a0. PMID 1731226.
- ↑ Chang WS. and Harper PL. (1997). "Commercial antithrombin concentrate contains inactive L-forms of antithrombin". Thromb. Haemost. 77 (2): 323–328. PMID 9157590.
- ↑ Wardell MR, Chang WS.; et al. (1997). "Preparative induction and characterization of L-antithrombin: a structural homologue of latent plasminogen activator inhibitor-1". Biochemistry. 36 (42): 13133–13142. doi:10.1021/bi970664u. PMID 9335576.
- ↑ Carrell RW, Huntington JA.; et al. (2001). "The conformational basis of thrombosis". Thromb. Haemost. 86 (1): 14–22. PMID 11487000.
- ↑ 37.0 37.1 37.2 Zhou A, Huntington JA. and Carrell RW. (1999). "Formation of the antithrombin heterodimer in vivo and the onset of thrombosis". Blood. 94 (10): 3388–3396. PMID 10552948.
- ↑ van Boven HH and Lane DA. (1997). "Antithrombin and its inherited deficiency states". Semin. Hematol. 34 (3): 188–204. PMID 9241705.
- ↑ Maclean PS and Tait RC. (2007). "Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options". Drugs. 67 (10): 1429–1440. PMID 17600391.
- ↑ 40.0 40.1 40.2 Lane DA, Olds RJ and Thein SL. (1994). "Antithrombin III: summary of first database update". Nucleic Acids Res. 22 (17): 3556–3559. PMID 7937056.
- ↑ Lane DA, Ireland H.; et al. (1991). "Antithrombin III: a database of mutations". Thromb. Haemost. 66 (6): 657–661. PMID 1796410.
- ↑ Picard V, Nowak-Göttl U.; et al. (2006). "Molecular bases of antithrombin deficiency: twenty-two novel mutations in the antithrombin gene". Hum. Mutat. 27 (6): 600. PMID 16705712.
External links
- Antithrombin+III at the US National Library of Medicine Medical Subject Headings (MeSH)