Anakinra: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |||
{{VP}} | {{VP}} | ||
Revision as of 15:11, 3 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
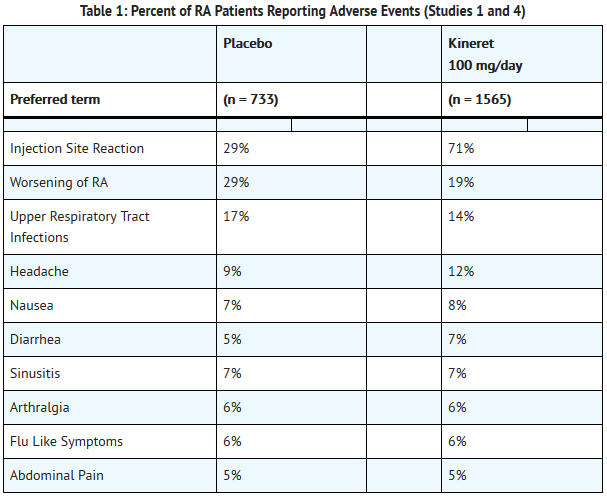
Anakinra is an interleukin-1 receptor antagonist that is FDA approved for the {{{indicationType}}} of rheumatoid arthritis (RA)and cryopyrin-associated periodic syndromes (CAPS). Common adverse reactions include upper respiratory tract infection, headache, nausea, diarrhea, sinusitis, arthralgia, flu like-symptoms, and abdominal pain.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Rheumatoid Arthritis
- The recommended dose of Kineret for the treatment of patients with rheumatoid arthritis is 100 mg/day administered daily by subcutaneous injection. Higher doses did not result in a higher response. The dose should be administered at approximately the same time every day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Anakinra in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anakinra in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Cryopyrin-Associated Periodic Syndromes (CAPS)
- The recommended starting dose of Kineret is 1-2 mg/kg for NOMID patients. The dose can be individually adjusted to a maximum of 8 mg/kg daily to control active inflammation.
- Adjust doses in 0.5 to 1.0 mg/kg increments. Once daily administration is generally recommended, but the dose may be split into twice daily administrations. Each syringe is intended for a single use. A new syringe must be used for each dose. Any unused portion after each dose should be discarded.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Anakinra in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anakinra in pediatric patients.
Contraindications
- Kineret is contraindicated in patients with known hypersensitivity to E coli-derived proteins, Kineret, or any components of the product.
Warnings
Precautions
- Serious Infections
- Kineret has been associated with an increased incidence of serious infections (2%) vs. Placebo (< 1%) in clinical trials in RA. Administration of Kineret in RA should be discontinued if a patient develops a serious infection. In Kineret treated NOMID patients the risk of a NOMID flare when discontinuing Kineret treatment should be weighed against the potential risk of continued treatment. Treatment with Kineret should not be initiated in patients with active infections. The safety and efficacy of Kineret in immunosuppressed patients or in patients with chronic infections have not been evaluated.
- Drugs that affect the immune system by blocking tumor necrosis factor (TNF) have been associated with an increased risk of reactivation of latent tuberculosis (TB). It is possible that taking drugs such as Kineret that blocks IL-1 increases the risk of TB or other atypical or opportunistic infections. Health care providers should follow current CDC guidelines both to evaluate for and to treat possible latent tuberculosis infections before initiating therapy with Kineret.
- Use With TNF Blocking Agents
- In a 24-week study of concurrent Kineret and etanercept therapy in RA patients, the rate of serious infections in the combination arm (7%) was higher than with etanercept alone (0%). The combination of Kineret and etanercept did not result in higher ACR response rates compared to etanercept alone [see clinical studies (14)]. Use of Kineret in combination with TNF blocking agents is not recommended.
- Hypersensitivity Reactions
- Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been reported with Kineret. If a severe hypersensitivity reaction occurs, administration of Kineret should be discontinued and appropriate therapy initiated.
- The needle cover of the prefilled syringe contains dry natural rubber (a derivative of latex), which may cause allergic reactions in individuals sensitive to latex.
- Immunosuppression
- The impact of treatment with Kineret on active and/or chronic infections and the development of malignancies is not known [see Adverse Reactions (6)].
- Immunizations
- In a placebo-controlled clinical trial (n = 126), no difference was detected in anti-tetanus antibody response between the Kineret and placebo treatment groups when the tetanus/diphtheria toxoids vaccine was administered concurrently with Kineret. No data are available on the effects of vaccination with other inactivated antigens in patients receiving Kineret. No data are available on either the effects of live vaccination or the secondary transmission of infection by live vaccines in patients receiving Kineret. Therefore, live vaccines should not be given concurrently with Kineret.
- Neutrophil Count
- Patients receiving Kineret may experience a decrease in neutrophil counts. Neutrophil counts should therefore be assessed prior to initiating Kineret treatment, and while receiving Kineret, monthly for 3 months, and thereafter quarterly for a period up to 1 year.
- In the placebo-controlled studies, 8% of RA patients receiving Kineret had decreases in neutrophil counts of at least one World Health Organization (WHO) toxicity grade compared with 2% in the placebo control group. Nine Kineret-treated patients (0.4%) experienced neutropenia (ANC < 1 x 109/L). This is discussed in more detail in the Adverse Reactions (6): Hematologic Events (6.1) section.
- In 43 NOMID patients followed for up to 60 months 2 patients experienced neutropenia that resolved over time during continued Kineret treatment. [see Adverse Reactions (6.2)]
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Anakinra in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Anakinra in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Anakinra in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Anakinra during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Anakinra with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Anakinra with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Anakinra with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Anakinra with respect to specific gender populations.
Race
There is no FDA guidance on the use of Anakinra with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Anakinra in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Anakinra in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Anakinra in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Anakinra in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Anakinra in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Anakinra in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Anakinra in the drug label.
Pharmacology
There is limited information regarding Anakinra Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Anakinra in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Anakinra in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Anakinra in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Anakinra in the drug label.
How Supplied
Storage
There is limited information regarding Anakinra Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Anakinra |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Anakinra |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Anakinra in the drug label.
Precautions with Alcohol
- Alcohol-Anakinra interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Anakinra |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Anakinra |Label Name=Anakinra11.png
}}
{{#subobject:
|Label Page=Anakinra |Label Name=Anakinra11.png
}}