Amlodipine and Benazepril: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
|drugClass= | |drugClass= | ||
combination of dihydropyridine [[calcium channel blocker]] (DHP CCB) and an [[angiotensin converting enzyme]] (ACE) inhibitor | |||
|indication= | |indication= | ||
[[hypertension]] | |||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
| Line 28: | Line 28: | ||
|adverseReactions= | |adverseReactions= | ||
[[cough]] and [[edema]] | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle= | |blackBoxWarningTitle= | ||
WARNING: FETAL TOXICITY | |||
|blackBoxWarningBody= | |blackBoxWarningBody= | ||
<i><span style="color:#FF0000;"> | <i><span style="color:#FF0000;"> </span></i> | ||
* | *When pregnancy is detected, discontinue Lotrel as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. | ||
<!--Adult Indications and Dosage--> | <!--Adult Indications and Dosage--> | ||
| Line 46: | Line 47: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Hypertension===== | ||
* Dosing Information | * Dosing Information | ||
:* | :* The recommended initial dose of Lotrel is one capsule of amlodipine 2.5 mg/benazepril 10 mg orally once daily. | ||
:*It is usually appropriate to begin therapy with Lotrel only after a patient has either (a) failed to achieve the desired antihypertensive effect with amlodipine or benazepril monotherapy, or (b) demonstrated inability to achieve adequate antihypertensive effect with amlodipine therapy without developing edema. | |||
:*The antihypertensive effect of Lotrel is largely attained within 2 weeks. If blood pressure remains uncontrolled, the dose may be titrated up to amlodipine 10 mg/benazepril 40 mg once daily. The dosing should be individualized and adjusted according to the patient’s clinical response. | |||
:*Amlodipine is an effective treatment of hypertension in once-daily doses of 2.5-10 mg while benazepril is effective in doses of 10-80 mg. In clinical trials of amlodipine/benazepril combination therapy using amlodipine doses of 2.5-10 mg and benazepril doses of 10-40 mg, the antihypertensive effects increased with increasing dose of amlodipine in all patient groups, and the effects increased with increasing dose of benazepril in nonblack groups. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 162: | Line 148: | ||
|contraindications= | |contraindications= | ||
* | *Do not co-administer [[aliskiren]] with [[angiotensin receptor blockers]], [[ACE inhibitors]], including Lotrel in patients with [[diabetes]]. | ||
*Lotrel is contraindicated in patients with a history of [[angioedema]], with or without previous [[ACE inhibitor]] treatment, or patients who are [[hypersensitive]] to benazepril, to any other [[ACE inhibitor]], to [[amlodipine]], or to any of the excipients of Lotrel. | |||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings= | ||
====Precautions==== | ====Precautions==== | ||
* | *Anaphylactoid and Possibly Related Reactions | ||
:*Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including Lotrel) may be subject to a variety of adverse reactions, some of them serious. These reactions usually occur after one of the first few doses of the ACE inhibitor, but they sometimes do not appear until after months of therapy. Black patients receiving ACE inhibitors have a higher incidence of angioedema compared to nonblacks. | |||
:*Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with ACE inhibitors. In U.S. clinical trials, symptoms consistent with angioedema were seen in none of the subjects who received placebo and in about 0.5% of the subjects who received benazepril. Angioedema associated with laryngeal edema can be fatal. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, discontinue treatment with Lotrel and treat immediately. When involvement of the tongue, glottis, or larynx appears likely to cause airway obstruction, appropriate therapy, e.g., administer subcutaneous epinephrine injection 1:1000 (0.3-0.5 mL), promptly [see Adverse Reactions (6)]. | |||
:*Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain. | |||
:*Anaphylactoid Reactions During Desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge. | |||
:*Anaphylactoid Reactions During Membrane Exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption. | |||
*Increased Angina and/or Myocardial Infarction | |||
:*Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease. | |||
*Patients with Aortic and Mitral Valve Stenosis, Obstructive Hypertrophic Cardiomyopathy | |||
:*As with all other vasodilators, special caution is required when using amlodipine in patients suffering from aortic or mitral stenosis, or obstructive hypertrophic cardiomyopathy. | |||
*Hypotension | |||
:*Lotrel can cause symptomatic hypotension. Symptomatic hypotension is most likely to occur in patients who have been volume or salt depleted as a result of diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Volume and/or salt depletion should be corrected before starting therapy with benazepril. If hypotension occurs, the patient should be placed in the supine position and if necessary given physiological saline i.v. Treatment with benazepril can be continued once blood pressure and volume have returned to normal. | |||
:*In patients with congestive heart failure, with or without associated renal insufficiency, ACE inhibitor therapy may cause excessive hypotension, which may be associated with oliguria, azotemia, and (rarely) with acute renal failure and death. In such patients, start Lotrel therapy under close medical supervision; follow closely for the first 2 weeks of treatment and whenever the dose of the benazepril component is increased or a diuretic is added or its dose increased. | |||
:*Symptomatic hypotension is also possible in patients with severe aortic stenosis. | |||
*Fetal Toxicity | |||
:*Pregnancy Category D | |||
:*Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Lotrel as soon as possible [see Use in Specific Populations (8.1)]. | |||
*Hepatitis and Hepatic Failure | |||
:*There have been rare reports of predominantly cholestatic hepatitis and isolated cases of acute liver failure, some of them fatal, in patients on ACE inhibitors. The mechanism is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevation of hepatic enzymes should discontinue the ACE inhibitor and be kept under medical surveillance. | |||
*Impaired Renal Function | |||
:*Monitor renal function periodically in patients treated with Lotrel. Changes in renal function, including acute renal failure, can be caused by drugs that affect the renin-angiotensin system. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, severe heart failure, post-myocardial infarction or volume depletion) or who are on NSAIDS or angiotensin receptor blockers may be at particular risk of developing acute renal failure on Lotrel. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on Lotrel. | |||
*Hyperkalemia | |||
:*Monitor serum potassium periodically in patients receiving Lotrel. Drugs that affect the renin-angiotensin system can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes. In U.S. placebo-controlled trials of Lotrel, hyperkalemia (serum potassium at least 0.5 mEq/L greater than the upper limit of normal) not present at baseline occurred in approximately 1.5% of hypertensive patients receiving Lotrel. Increases in serum potassium were generally reversible. | |||
*Cough | |||
:*Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, generally resolving after discontinuation of therapy. Consider ACE inhibitor-induced cough in the differential diagnosis of cough. | |||
*Surgery/Anesthesia | |||
:*In patients undergoing surgery or during anesthesia with agents that produce hypotension, benazepril will block the angiotensin II formation that could otherwise occur secondary to compensatory renin release. Hypotension that occurs as a result of this mechanism can be corrected by volume expansion. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates. | |||
*Lotrel has been evaluated for safety in over 2,991 patients with hypertension; over 500 of these patients were treated for at least 6 months, and over 400 were treated for more than 1 year. | |||
*In a pooled analysis of 5 placebo-controlled trials involving Lotrel doses up to 5/20, the reported side effects were generally mild and transient, and there was no relationship between side effects and age, sex, race, or duration of therapy. Discontinuation of therapy due to side effects was required in approximately 4% of patients treated with Lotrel and in 3% of patients treated with placebo. | |||
*The most common reasons for discontinuation of therapy with Lotrel in these studies were cough and edema (including angioedema). | |||
*The peripheral edema associated with amlodipine use is dose-dependent. When benazepril is added to a regimen of amlodipine, the incidence of edema is substantially reduced. | |||
*The addition of benazepril to a regimen of amlodipine should not be expected to provide additional antihypertensive effect in African-Americans. However, all patient groups benefit from the reduction in amlodipine-induced edema. | |||
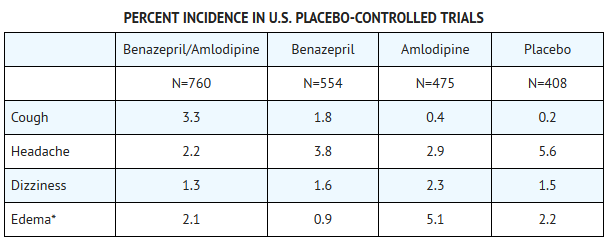
*The side effects considered possibly or probably related to study drug that occurred in these trials in more than 1% of patients treated with Lotrel are shown in the table below. Cough was the only adverse event with at least possible relationship to treatment that was more common on Lotrel (3.3%) than on placebo (0.2%). | |||
T1 | |||
*Edema refers to all edema, such as dependent edema, angioedema, facial edema. | |||
*The incidence of edema was greater in patients treated with amlodipine monotherapy (5.1%) than in patients treated with Lotrel (2.1%) or placebo (2.2%). | |||
*Other side effects considered possibly or probably related to study drug that occurred in U.S. placebo-controlled trials of patients treated with Lotrel or in postmarketing experience were the following: | |||
=====Body as a Whole===== | |||
===== | Asthenia and fatigue. | ||
=====CNS===== | |||
Insomnia, nervousness, anxiety, tremor, and decreased libido. | |||
=====Dermatologic===== | |||
Flushing, hot flashes, rash, skin nodule, and dermatitis. | |||
===== | =====Digestive===== | ||
Dry mouth, nausea, abdominal pain, constipation, diarrhea, dyspepsia, and esophagitis. | |||
=====Hematologic===== | |||
Neutropenia | |||
===== | =====Metabolic and Nutritional===== | ||
Hypokalemia. | |||
=====Musculoskeletal===== | |||
Back pain, musculoskeletal pain, cramps, and muscle cramps. | |||
=====Respiratory===== | =====Respiratory===== | ||
Pharyngitis. | |||
=====Urogenital===== | =====Urogenital===== | ||
Sexual problems such as impotence, and polyuria. | |||
*Monotherapies of benazepril and amlodipine have been evaluated for safety in clinical trials in over 6,000 and 11,000 patients, respectively. The observed adverse reactions to the monotherapies in these trials were similar to those seen in trials of Lotrel. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 250: | Line 265: | ||
|postmarketing= | |postmarketing= | ||
*Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
*In postmarketing experience with benazepril, there have been rare reports of Stevens-Johnson syndrome, pancreatitis, hemolytic anemia, pemphigus, thrombocytopenia, paresthesia, dysgeusia, orthostatic symptoms and hypotension, angina pectoris and arrhythmia, pruritus, photosensitivity reaction, arthralgia, arthritis, myalgia, BUN increase, serum creatinine increased, renal impairment, impaired vision, agranulocytosis, neutropenia. | |||
*Rare reports in association with use of amlodipine: gingival hyperplasia, tachycardia, jaundice, and hepatic enzyme elevations (mostly consistent with cholestasis severe enough to require hospitalization), leucocytopenia, allergic reaction, hyperglycemia, dysgeusia, hypoesthesia, paresthesia, syncope, peripheral neuropathy, hypertonia, visual impairment, diplopia, hypotension, vasculitis, rhinitis, gastritis, hyperhidrosis, pruritis, skin discoloration, urticaria, erythema multiform, muscle spasms, arthralgia, micturition disorder, nocturia, erectile dysfunction, malaise, weight decrease or gain. | |||
*Other potentially important adverse experiences attributed to other ACE inhibitors and calcium channel blockers include: eosinophilic pneumonitis (ACE inhibitors) and gynecomastia (CCBs). Other infrequently reported events included chest pain, ventricular extrasystole, gout, neuritis, tinnitus, alopecia, upper respiratory tract infection, palpitations and somnolence. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
=====Amlodipine===== | |||
*Simvastatin | |||
:*Co-administration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily. | |||
*CYP3A4 Inhibitors | |||
:*Co-administration with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with CYP3A4 inhibitors to determine the need for dose adjustment. | |||
*CYP3A4 Inducers | |||
:*No information is available on the quantitative effects of CYP3A4 inducers on amlodipine. Blood pressure should be monitored when amlodipine is co-administered with CYP3A4 inducers. | |||
===== | =====Benazepril===== | ||
*Potassium Supplements and Potassium-Sparing Diuretics | |||
:*Benazepril can attenuate potassium loss caused by thiazide diuretics. Potassium-sparing diuretics (spironolactone, amiloride, triamterene, and others) or potassium supplements can increase the risk of hyperkalemia. If concomitant use of such agents is indicated, the patient’s serum potassium should be monitored frequently. | |||
*Lithium | |||
:*Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium. When coadministering Lotrel and lithium, frequent monitoring of serum lithium levels is recommended. | |||
*Gold | |||
:*Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy. | |||
*Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors) | |||
:*In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including benazepril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving benazepril and NSAID therapy. | |||
:*The antihypertensive effect of ACE inhibitors, including benazepril, may be attenuated by NSAIDs. | |||
*Antidiabetic agents | |||
:*In rare cases, diabetic patients receiving an ACE inhibitor (including benazepril) concomitantly with insulin or oral antidiabetics may develop hypoglycemia. Such patients should therefore be advised about the possibility of hypoglycemic reactions, and should be monitored accordingly. | |||
*Dual Blockade of the Renin-Angiotensin System (RAS) | |||
:*Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on Lotrel and other agents that block the RAS. | |||
:*Do not co-administer aliskiren with Lotrel in patients with diabetes. Avoid use of aliskiren with Lotrel in patients with renal impairment (GFR <60 ml/min). | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category D''' | |||
*Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Lotrel as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. | |||
*In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Lotrel, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Lotrel for hypotension, oliguria, and hyperkalemia. | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
*The effect of Lotrel on labor and delivery has not been studied. | |||
|useInNursing= | |||
*Minimal amounts of unchanged benazepril and of benazeprilat are excreted into the breast milk of lactating women treated with benazepril, so that a newborn child ingesting nothing but breast milk would receive less than 0.1% of the maternal doses of benazepril and benazeprilat. | |||
*It is not known whether amlodipine is excreted in human milk. Nursing or drug should be discontinued. | |||
|useInPed= | |||
*Neonates with a history of in utero exposure to Lotrel: | |||
*If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Benazepril, which crosses the placenta, can theoretically be removed from the neonatal circulation by these means; there are occasional reports of benefit from these maneuvers, but experience is limited. | |||
|useInGeri= | |||
| | |||
*In geriatrics, exposure to amlodipine is increased, thus consider lower initial doses of Lotrel [see Clinical Pharmacology (12.3)]. | |||
*Of the total number of patients who received Lotrel in U.S. clinical studies of Lotrel, over 19% were 65 or older while about 2% were 75 or older. Overall differences in effectiveness or safety were not observed between these patients and younger patients. Clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. | |||
|useInGender= | |useInGender= | ||
| Line 340: | Line 353: | ||
|useInRenalImpair= | |useInRenalImpair= | ||
*In patients with severe renal impairment systemic exposure to benazepril is increased. The recommended dose of benazepril in this subgroup is 5 mg which is not an available strength with Lotrel. Lotrel is not recommended in patients with severe renal impairment. No dose adjustment of Lotrel is needed in patients with mild or moderate impairment of renal function. | |||
|useInHepaticImpair= | |useInHepaticImpair= | ||
*Exposure to amlodipine is increased in patients with hepatic insufficiency, thus consider using lower doses of Lotrel. | |||
|useInReproPotential= | |useInReproPotential= | ||
| Line 356: | Line 371: | ||
* Oral | * Oral | ||
|monitoring= | |monitoring= | ||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
| Line 379: | Line 390: | ||
====Signs and Symptoms==== | ====Signs and Symptoms==== | ||
* | *Only a few cases of human overdose with amlodipine have been reported. One patient was asymptomatic after a 250-mg ingestion; another, who combined 70 mg of amlodipine with an unknown large quantity of a benzodiazepine, developed refractory shock and died. | ||
*Human overdoses with any combination of amlodipine and benazepril have not been reported. In scattered reports of human overdoses with benazepril and other ACE inhibitors, there are no reports of death. | |||
*Other clinical manifestations of overdose should be managed symptomatically based on modern methods of intensive care. | |||
*To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison-Control Center. Telephone numbers of certified poison-control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdose, consider the possibilities of multiple-drug overdoses, drug-drug interactions, and unusual drug kinetics in your patient. | |||
*The most likely effect of overdose with Lotrel is vasodilation, with consequent hypotension and tachycardia. Simple repletion of central fluid volume (Trendelenburg positioning, infusion of crystalloids) may be sufficient therapy, but pressor agents (norepinephrine or high-dose dopamine) may be required. With abrupt return of peripheral vascular tone, overdoses of other dihydropyridine calcium channel blockers have sometimes progressed to pulmonary edema, and patients must be monitored for this complication. | |||
*Analyses of bodily fluids for concentrations of amlodipine, benazepril, or their metabolites are not widely available. Such analyses are, in any event, not known to be of value in therapy or prognosis. | |||
*No data are available to suggest physiologic maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of amlodipine, benazepril, or their metabolites. Benazeprilat is only slightly dialyzable; attempted clearance of amlodipine by hemodialysis or hemo-perfusion has not been reported, but amlodipine’s high protein binding makes it unlikely that these interventions will be of value. | |||
====Management==== | ====Management==== | ||
* | *Patients should be admitted to hospital and, generally, should be managed in an intensive care setting, with continuous monitoring of cardiac function, blood gases, and blood biochemistry. Emergency supportive measures such as artificial ventilation or cardiac pacing should be instituted if appropriate. | ||
*In the event of a potentially life-threatening oral overdose, use induction of vomiting or gastric lavage and/or activated charcoal to remove the drug from the gastrointestinal tract (only if presented within 1 hour after ingestion of Lotrel). | |||
*Angiotensin II could presumably serve as a specific antagonist-antidote to benazepril, but angiotensin II is essentially unavailable outside of scattered research laboratories. | |||
===Chronic Overdose=== | ===Chronic Overdose=== | ||
Revision as of 13:47, 27 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
|
Overview
Amlodipine and Benazepril is a combination of dihydropyridine calcium channel blocker (DHP CCB) and an angiotensin converting enzyme (ACE) inhibitor that is FDA approved for the {{{indicationType}}} of hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include cough and edema.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing Information
- The recommended initial dose of Lotrel is one capsule of amlodipine 2.5 mg/benazepril 10 mg orally once daily.
- It is usually appropriate to begin therapy with Lotrel only after a patient has either (a) failed to achieve the desired antihypertensive effect with amlodipine or benazepril monotherapy, or (b) demonstrated inability to achieve adequate antihypertensive effect with amlodipine therapy without developing edema.
- The antihypertensive effect of Lotrel is largely attained within 2 weeks. If blood pressure remains uncontrolled, the dose may be titrated up to amlodipine 10 mg/benazepril 40 mg once daily. The dosing should be individualized and adjusted according to the patient’s clinical response.
- Amlodipine is an effective treatment of hypertension in once-daily doses of 2.5-10 mg while benazepril is effective in doses of 10-80 mg. In clinical trials of amlodipine/benazepril combination therapy using amlodipine doses of 2.5-10 mg and benazepril doses of 10-40 mg, the antihypertensive effects increased with increasing dose of amlodipine in all patient groups, and the effects increased with increasing dose of benazepril in nonblack groups.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Amlodipine and Benazepril in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Amlodipine and Benazepril in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Amlodipine and Benazepril in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Amlodipine and Benazepril in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Amlodipine and Benazepril in pediatric patients.
Contraindications
- Do not co-administer aliskiren with angiotensin receptor blockers, ACE inhibitors, including Lotrel in patients with diabetes.
- Lotrel is contraindicated in patients with a history of angioedema, with or without previous ACE inhibitor treatment, or patients who are hypersensitive to benazepril, to any other ACE inhibitor, to amlodipine, or to any of the excipients of Lotrel.
Warnings
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
|
Precautions
- Anaphylactoid and Possibly Related Reactions
- Presumably because angiotensin-converting enzyme inhibitors affect the metabolism of eicosanoids and polypeptides, including endogenous bradykinin, patients receiving ACE inhibitors (including Lotrel) may be subject to a variety of adverse reactions, some of them serious. These reactions usually occur after one of the first few doses of the ACE inhibitor, but they sometimes do not appear until after months of therapy. Black patients receiving ACE inhibitors have a higher incidence of angioedema compared to nonblacks.
- Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with ACE inhibitors. In U.S. clinical trials, symptoms consistent with angioedema were seen in none of the subjects who received placebo and in about 0.5% of the subjects who received benazepril. Angioedema associated with laryngeal edema can be fatal. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, discontinue treatment with Lotrel and treat immediately. When involvement of the tongue, glottis, or larynx appears likely to cause airway obstruction, appropriate therapy, e.g., administer subcutaneous epinephrine injection 1:1000 (0.3-0.5 mL), promptly [see Adverse Reactions (6)].
- Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
- Anaphylactoid Reactions During Desensitization: Two patients undergoing desensitizing treatment with hymenoptera venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions. In the same patients, these reactions were avoided when ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
- Anaphylactoid Reactions During Membrane Exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
- Increased Angina and/or Myocardial Infarction
- Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease.
- Patients with Aortic and Mitral Valve Stenosis, Obstructive Hypertrophic Cardiomyopathy
- As with all other vasodilators, special caution is required when using amlodipine in patients suffering from aortic or mitral stenosis, or obstructive hypertrophic cardiomyopathy.
- Hypotension
- Lotrel can cause symptomatic hypotension. Symptomatic hypotension is most likely to occur in patients who have been volume or salt depleted as a result of diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Volume and/or salt depletion should be corrected before starting therapy with benazepril. If hypotension occurs, the patient should be placed in the supine position and if necessary given physiological saline i.v. Treatment with benazepril can be continued once blood pressure and volume have returned to normal.
- In patients with congestive heart failure, with or without associated renal insufficiency, ACE inhibitor therapy may cause excessive hypotension, which may be associated with oliguria, azotemia, and (rarely) with acute renal failure and death. In such patients, start Lotrel therapy under close medical supervision; follow closely for the first 2 weeks of treatment and whenever the dose of the benazepril component is increased or a diuretic is added or its dose increased.
- Symptomatic hypotension is also possible in patients with severe aortic stenosis.
- Fetal Toxicity
- Pregnancy Category D
- Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Lotrel as soon as possible [see Use in Specific Populations (8.1)].
- Hepatitis and Hepatic Failure
- There have been rare reports of predominantly cholestatic hepatitis and isolated cases of acute liver failure, some of them fatal, in patients on ACE inhibitors. The mechanism is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevation of hepatic enzymes should discontinue the ACE inhibitor and be kept under medical surveillance.
- Impaired Renal Function
- Monitor renal function periodically in patients treated with Lotrel. Changes in renal function, including acute renal failure, can be caused by drugs that affect the renin-angiotensin system. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, severe heart failure, post-myocardial infarction or volume depletion) or who are on NSAIDS or angiotensin receptor blockers may be at particular risk of developing acute renal failure on Lotrel. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on Lotrel.
- Hyperkalemia
- Monitor serum potassium periodically in patients receiving Lotrel. Drugs that affect the renin-angiotensin system can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes. In U.S. placebo-controlled trials of Lotrel, hyperkalemia (serum potassium at least 0.5 mEq/L greater than the upper limit of normal) not present at baseline occurred in approximately 1.5% of hypertensive patients receiving Lotrel. Increases in serum potassium were generally reversible.
- Cough
- Presumably due to the inhibition of the degradation of endogenous bradykinin, persistent nonproductive cough has been reported with all ACE inhibitors, generally resolving after discontinuation of therapy. Consider ACE inhibitor-induced cough in the differential diagnosis of cough.
- Surgery/Anesthesia
- In patients undergoing surgery or during anesthesia with agents that produce hypotension, benazepril will block the angiotensin II formation that could otherwise occur secondary to compensatory renin release. Hypotension that occurs as a result of this mechanism can be corrected by volume expansion.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
- Lotrel has been evaluated for safety in over 2,991 patients with hypertension; over 500 of these patients were treated for at least 6 months, and over 400 were treated for more than 1 year.
- In a pooled analysis of 5 placebo-controlled trials involving Lotrel doses up to 5/20, the reported side effects were generally mild and transient, and there was no relationship between side effects and age, sex, race, or duration of therapy. Discontinuation of therapy due to side effects was required in approximately 4% of patients treated with Lotrel and in 3% of patients treated with placebo.
- The most common reasons for discontinuation of therapy with Lotrel in these studies were cough and edema (including angioedema).
- The peripheral edema associated with amlodipine use is dose-dependent. When benazepril is added to a regimen of amlodipine, the incidence of edema is substantially reduced.
- The addition of benazepril to a regimen of amlodipine should not be expected to provide additional antihypertensive effect in African-Americans. However, all patient groups benefit from the reduction in amlodipine-induced edema.
- The side effects considered possibly or probably related to study drug that occurred in these trials in more than 1% of patients treated with Lotrel are shown in the table below. Cough was the only adverse event with at least possible relationship to treatment that was more common on Lotrel (3.3%) than on placebo (0.2%).
T1
- Edema refers to all edema, such as dependent edema, angioedema, facial edema.
- The incidence of edema was greater in patients treated with amlodipine monotherapy (5.1%) than in patients treated with Lotrel (2.1%) or placebo (2.2%).
- Other side effects considered possibly or probably related to study drug that occurred in U.S. placebo-controlled trials of patients treated with Lotrel or in postmarketing experience were the following:
Body as a Whole
Asthenia and fatigue.
CNS
Insomnia, nervousness, anxiety, tremor, and decreased libido.
Dermatologic
Flushing, hot flashes, rash, skin nodule, and dermatitis.
Digestive
Dry mouth, nausea, abdominal pain, constipation, diarrhea, dyspepsia, and esophagitis.
Hematologic
Neutropenia
Metabolic and Nutritional
Hypokalemia.
Musculoskeletal
Back pain, musculoskeletal pain, cramps, and muscle cramps.
Respiratory
Pharyngitis.
Urogenital
Sexual problems such as impotence, and polyuria.
- Monotherapies of benazepril and amlodipine have been evaluated for safety in clinical trials in over 6,000 and 11,000 patients, respectively. The observed adverse reactions to the monotherapies in these trials were similar to those seen in trials of Lotrel.
Postmarketing Experience
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- In postmarketing experience with benazepril, there have been rare reports of Stevens-Johnson syndrome, pancreatitis, hemolytic anemia, pemphigus, thrombocytopenia, paresthesia, dysgeusia, orthostatic symptoms and hypotension, angina pectoris and arrhythmia, pruritus, photosensitivity reaction, arthralgia, arthritis, myalgia, BUN increase, serum creatinine increased, renal impairment, impaired vision, agranulocytosis, neutropenia.
- Rare reports in association with use of amlodipine: gingival hyperplasia, tachycardia, jaundice, and hepatic enzyme elevations (mostly consistent with cholestasis severe enough to require hospitalization), leucocytopenia, allergic reaction, hyperglycemia, dysgeusia, hypoesthesia, paresthesia, syncope, peripheral neuropathy, hypertonia, visual impairment, diplopia, hypotension, vasculitis, rhinitis, gastritis, hyperhidrosis, pruritis, skin discoloration, urticaria, erythema multiform, muscle spasms, arthralgia, micturition disorder, nocturia, erectile dysfunction, malaise, weight decrease or gain.
- Other potentially important adverse experiences attributed to other ACE inhibitors and calcium channel blockers include: eosinophilic pneumonitis (ACE inhibitors) and gynecomastia (CCBs). Other infrequently reported events included chest pain, ventricular extrasystole, gout, neuritis, tinnitus, alopecia, upper respiratory tract infection, palpitations and somnolence.
Drug Interactions
Amlodipine
- Simvastatin
- Co-administration of simvastatin with amlodipine increases the systemic exposure of simvastatin. Limit the dose of simvastatin in patients on amlodipine to 20 mg daily.
- CYP3A4 Inhibitors
- Co-administration with CYP3A inhibitors (moderate and strong) results in increased systemic exposure to amlodipine and may require dose reduction. Monitor for symptoms of hypotension and edema when amlodipine is co-administered with CYP3A4 inhibitors to determine the need for dose adjustment.
- CYP3A4 Inducers
- No information is available on the quantitative effects of CYP3A4 inducers on amlodipine. Blood pressure should be monitored when amlodipine is co-administered with CYP3A4 inducers.
Benazepril
- Potassium Supplements and Potassium-Sparing Diuretics
- Benazepril can attenuate potassium loss caused by thiazide diuretics. Potassium-sparing diuretics (spironolactone, amiloride, triamterene, and others) or potassium supplements can increase the risk of hyperkalemia. If concomitant use of such agents is indicated, the patient’s serum potassium should be monitored frequently.
- Lithium
- Increased serum lithium levels and symptoms of lithium toxicity have been reported in patients receiving ACE inhibitors during therapy with lithium. When coadministering Lotrel and lithium, frequent monitoring of serum lithium levels is recommended.
- Gold
- Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy.
- Non-Steroidal Anti-Inflammatory Agents including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
- In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including benazepril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving benazepril and NSAID therapy.
- The antihypertensive effect of ACE inhibitors, including benazepril, may be attenuated by NSAIDs.
- Antidiabetic agents
- In rare cases, diabetic patients receiving an ACE inhibitor (including benazepril) concomitantly with insulin or oral antidiabetics may develop hypoglycemia. Such patients should therefore be advised about the possibility of hypoglycemic reactions, and should be monitored accordingly.
- Dual Blockade of the Renin-Angiotensin System (RAS)
- Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or aliskiren is associated with increased risks of hypotension, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy. Closely monitor blood pressure, renal function and electrolytes in patients on Lotrel and other agents that block the RAS.
- Do not co-administer aliskiren with Lotrel in patients with diabetes. Avoid use of aliskiren with Lotrel in patients with renal impairment (GFR <60 ml/min).
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Lotrel as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus.
- In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Lotrel, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe infants with histories of in utero exposure to Lotrel for hypotension, oliguria, and hyperkalemia.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amlodipine and Benazepril in women who are pregnant.
Labor and Delivery
- The effect of Lotrel on labor and delivery has not been studied.
Nursing Mothers
- Minimal amounts of unchanged benazepril and of benazeprilat are excreted into the breast milk of lactating women treated with benazepril, so that a newborn child ingesting nothing but breast milk would receive less than 0.1% of the maternal doses of benazepril and benazeprilat.
- It is not known whether amlodipine is excreted in human milk. Nursing or drug should be discontinued.
Pediatric Use
- Neonates with a history of in utero exposure to Lotrel:
- If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function. Benazepril, which crosses the placenta, can theoretically be removed from the neonatal circulation by these means; there are occasional reports of benefit from these maneuvers, but experience is limited.
Geriatic Use
- In geriatrics, exposure to amlodipine is increased, thus consider lower initial doses of Lotrel [see Clinical Pharmacology (12.3)].
- Of the total number of patients who received Lotrel in U.S. clinical studies of Lotrel, over 19% were 65 or older while about 2% were 75 or older. Overall differences in effectiveness or safety were not observed between these patients and younger patients. Clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Amlodipine and Benazepril with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amlodipine and Benazepril with respect to specific racial populations.
Renal Impairment
- In patients with severe renal impairment systemic exposure to benazepril is increased. The recommended dose of benazepril in this subgroup is 5 mg which is not an available strength with Lotrel. Lotrel is not recommended in patients with severe renal impairment. No dose adjustment of Lotrel is needed in patients with mild or moderate impairment of renal function.
Hepatic Impairment
- Exposure to amlodipine is increased in patients with hepatic insufficiency, thus consider using lower doses of Lotrel.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amlodipine and Benazepril in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amlodipine and Benazepril in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Amlodipine and Benazepril in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Amlodipine and Benazepril in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Only a few cases of human overdose with amlodipine have been reported. One patient was asymptomatic after a 250-mg ingestion; another, who combined 70 mg of amlodipine with an unknown large quantity of a benzodiazepine, developed refractory shock and died.
- Human overdoses with any combination of amlodipine and benazepril have not been reported. In scattered reports of human overdoses with benazepril and other ACE inhibitors, there are no reports of death.
- Other clinical manifestations of overdose should be managed symptomatically based on modern methods of intensive care.
- To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison-Control Center. Telephone numbers of certified poison-control centers are listed in the Physicians’ Desk Reference (PDR). In managing overdose, consider the possibilities of multiple-drug overdoses, drug-drug interactions, and unusual drug kinetics in your patient.
- The most likely effect of overdose with Lotrel is vasodilation, with consequent hypotension and tachycardia. Simple repletion of central fluid volume (Trendelenburg positioning, infusion of crystalloids) may be sufficient therapy, but pressor agents (norepinephrine or high-dose dopamine) may be required. With abrupt return of peripheral vascular tone, overdoses of other dihydropyridine calcium channel blockers have sometimes progressed to pulmonary edema, and patients must be monitored for this complication.
- Analyses of bodily fluids for concentrations of amlodipine, benazepril, or their metabolites are not widely available. Such analyses are, in any event, not known to be of value in therapy or prognosis.
- No data are available to suggest physiologic maneuvers (e.g., maneuvers to change the pH of the urine) that might accelerate elimination of amlodipine, benazepril, or their metabolites. Benazeprilat is only slightly dialyzable; attempted clearance of amlodipine by hemodialysis or hemo-perfusion has not been reported, but amlodipine’s high protein binding makes it unlikely that these interventions will be of value.
Management
- Patients should be admitted to hospital and, generally, should be managed in an intensive care setting, with continuous monitoring of cardiac function, blood gases, and blood biochemistry. Emergency supportive measures such as artificial ventilation or cardiac pacing should be instituted if appropriate.
- In the event of a potentially life-threatening oral overdose, use induction of vomiting or gastric lavage and/or activated charcoal to remove the drug from the gastrointestinal tract (only if presented within 1 hour after ingestion of Lotrel).
- Angiotensin II could presumably serve as a specific antagonist-antidote to benazepril, but angiotensin II is essentially unavailable outside of scattered research laboratories.
Chronic Overdose
There is limited information regarding Chronic Overdose of Amlodipine and Benazepril in the drug label.
Pharmacology
There is limited information regarding Amlodipine and Benazepril Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Amlodipine and Benazepril in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Amlodipine and Benazepril in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Amlodipine and Benazepril in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Amlodipine and Benazepril in the drug label.
How Supplied
Storage
There is limited information regarding Amlodipine and Benazepril Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Amlodipine and Benazepril |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amlodipine and Benazepril |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Amlodipine and Benazepril in the drug label.
Precautions with Alcohol
- Alcohol-Amlodipine and Benazepril interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Amlodipine and Benazepril |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Amlodipine and Benazepril |Label Name=Amlodipine and Benazepril11.png
}}
{{#subobject:
|Label Page=Amlodipine and Benazepril |Label Name=Amlodipine and Benazepril11.png
}}