Aminosalicylic acid
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 50–60% |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
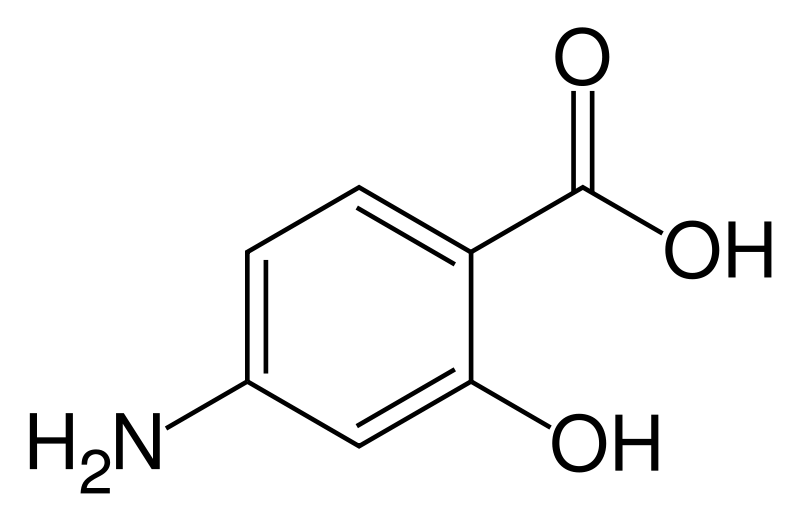
| Formula | C7H7NO3 |
| Molar mass | 153.135 g/mol |
| 3D model (JSmol) | |
| Melting point | 150.5 °C (302.9 °F) |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Aminosalicylic acid, also known as para-aminosalicylic acid, p-aminosalicylic acid or 4-aminosalicylic acid; abbreviated 4-ASA, PAS or P, is an antibiotic used to treat tuberculosis. It has been use for over forty years in the treatment of inflammatory bowel diseases (IBDs), where it has shown greater potency in Crohn's disease. It is thought to act via NF-κB (nuclear factor-kappa B) inhibition and free radical scavenging.
Aminosalicylic acid is sold in the United States by Jacobus Pharmaceutical as Paser. Mesalazine (5-aminosalicylic acid) is a closely related compound that also has medical uses.
Medical uses
Aminosalicylic acid was introduced to clinical use in 1948. It was the second antibiotic found to be effective in the treatment of tuberculosis (after streptomycin).
Its potency is less than that of the current five first-line drugs (isoniazid, rifampicin, ethambutol, pyrazinamide and streptomycin) for treating tuberculosis. It still has a role to play in the treatment of multidrug-resistant tuberculosis. Aminosalicylic acid is always used in combination with other anti-TB drugs and is never used on its own.
The dose when treating tuberculosis is 150mg/kg/day divided into two to four daily doses; the usual adult dose is therefore approximately 2 to 4g four times a day. It is sold in the US as Paser which comes in the form of 4g packets of delayed-release granules that have to be measured out with a scoop. The drug should be taken with acid food or drink (orange, apple or tomato juice).
Aminosalicylic acid used to be available in a combination formula with isoniazid called Pasinah.
Aminosalicylic acid has also used in the treatment of inflammatory bowel diseases, but has been superseded by other drugs such as sulfasalazine and mesalazine.
Pharmacology
It has a molecular weight of 153.14. With heat, aminosalicylic acid is decarboxylated to produce CO2 and m-aminophenol. Granules are tan in colour and must be refrigerated: they turn dark brown or purple when kept at room temperature for prolonged periods and should not be taken.
Aminosalicylic acid is in the FDA pregnancy category C. This means that it is not known whether it will harm an unborn baby.
Side effects
Gastrointestinal side-effects (nausea, vomiting, diarrhoea) are common; the delayed-release formulation is meant to help overcome this problem. It is also a cause of drug-induced hepatitis. Patients with glucose-6-phosphate dehydrogenase deficiency should avoid taking aminosalicylic acid as it causes haemolysis. Thyroid goitre is also a side-effect because aminosalicylic acid inhibits the synthesis of thyroid hormones.
There are drug interactions (in particular, phenytoin levels are increased).
History
PAS was discovered by the Swedish chemist Jörgen Lehmann while he followed through on published information that the tuberculosis bacterium avidly metabolized salicylic acid. Lehmann first tried PAS as an oral TB therapy late in 1944. The first patient made a dramatic recovery. The drug proved better than streptomycin, which had nerve toxicity and to which TB could easily develop resistance.
Late in the 1940s, researchers at Britain's Medical Research Council demonstrated that combined treatment with streptomycin and PAS was superior to either drug alone.
External links
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Tuberculosis
- Antibiotics
- Pulmonology