Amidine
Amidines are a class of oxoacid derivatives.
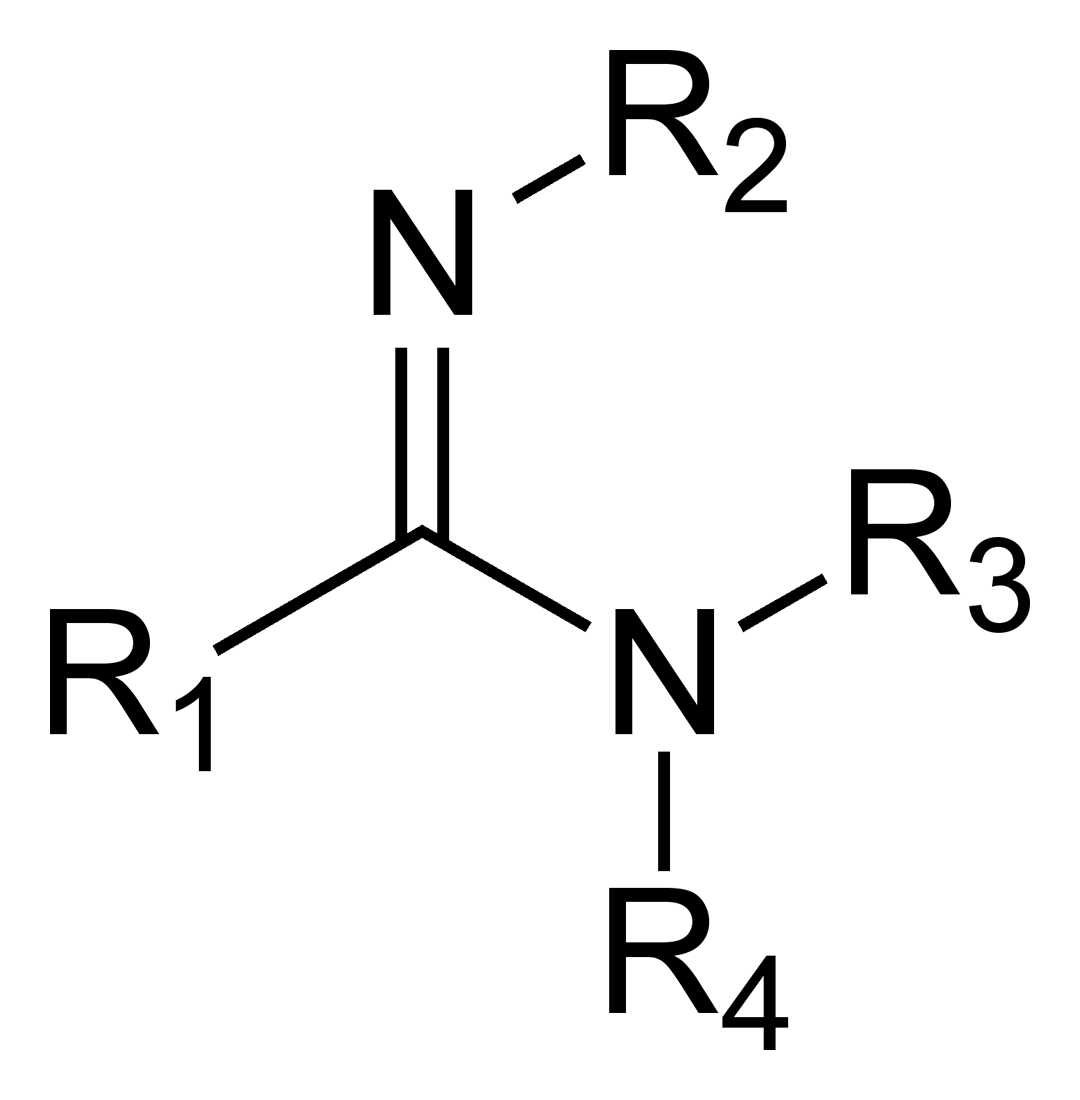
The oxoacid from which an amidine is derived must be of the form RnE(=O)OH, where R is a substituent. The −OH group is replaced by an −NH2 group and the =O group is replaced by =NR, giving amidines the general structure RnE(=NR)NR2.

When the parent oxoacid is a carboxylic acid, the resulting amidine is a carboxamidine, and has the following general structure:
Carboxamidines are frequently referred to simply as amidines, as they are the most commonly-encountered type of amidine in organic chemistry. The simplest amidine is acetamidine, CH3C(=NH)NH2.
Examples of amidines include DBU and diminazene.