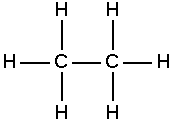
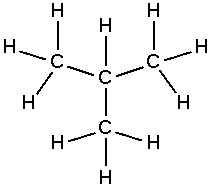
Aliphatic compound
In chemistry, aliphatic compounds are organic compounds in which carbon atoms are joined together in straight or branched chains or in rings, that can be either saturated or unsaturated, but not aromatic.[1] The simplest aliphatic compound is methane (CH4). Aliphatics include not only the fatty acids and other derivatives of paraffin hydrocarbons (alkanes), but also unsaturated compounds, including the alkenes (such as ethylene) and the alkynes (such as acetylene). The most frequently found non-carbon atoms bound to the carbon chain include hydrogen, oxygen, nitrogen, sulfur, and various halides.
Most aliphatic compounds have exothermic combustion reactions, thus allowing hydrocarbons such as methane to fuel Bunsen burners in the laboratory, while acetylene is used as torch fuel in pipe fitting.
Examples
See also
References
Template:Organic-compound-stub
ar:أليفاتي ca:Hidrocarbur alifàtic cs:Alifatický uhlovodík da:Alifatisk forbindelse de:Aliphatische Kohlenwasserstoffe id:Alifatik he:אליפטיות nl:Alifatische verbinding fi:Alifaattinen yhdiste sv:Alifatisk uk:Аліфатичні сполуки