Alaproclate
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
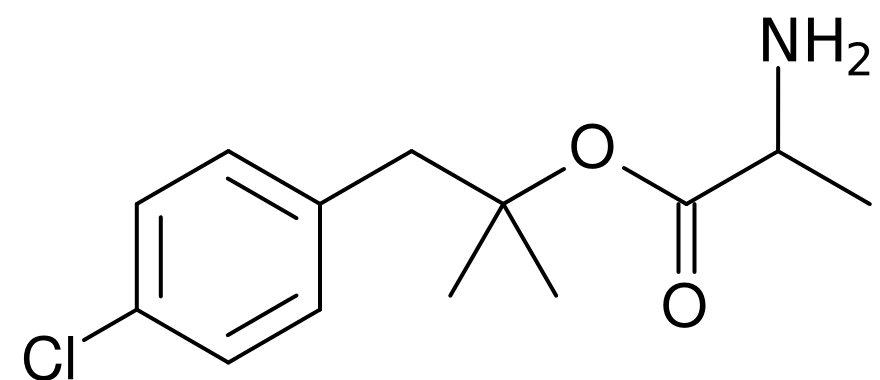
| Formula | C13H18ClNO2 |
| Molar mass | 255.740 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Alaproclate |
|
Articles |
|---|
|
Most recent articles on Alaproclate Most cited articles on Alaproclate |
|
Media |
|
Powerpoint slides on Alaproclate |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Alaproclate at Clinical Trials.gov Clinical Trials on Alaproclate at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Alaproclate
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Alaproclate Discussion groups on Alaproclate Patient Handouts on Alaproclate Directions to Hospitals Treating Alaproclate Risk calculators and risk factors for Alaproclate
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Alaproclate |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Alaproclate (GEA-654) is a psychoactive drug and research chemical that was being developed as an antidepressant by the Swedish pharmaceutical company Astra AB (now AstraZeneca) in the 1970s. It acts as a selective serotonin reuptake inhibitor (SSRI), and along with zimelidine and indalpine, was one of the first of its kind. Development was discontinued due to the observation of liver complications in rodent studies. Some studies have found that it acts as a noncompetitive NMDA antagonist, but does not have discriminative stimulus properties similar to phencyclidine.[1][2]
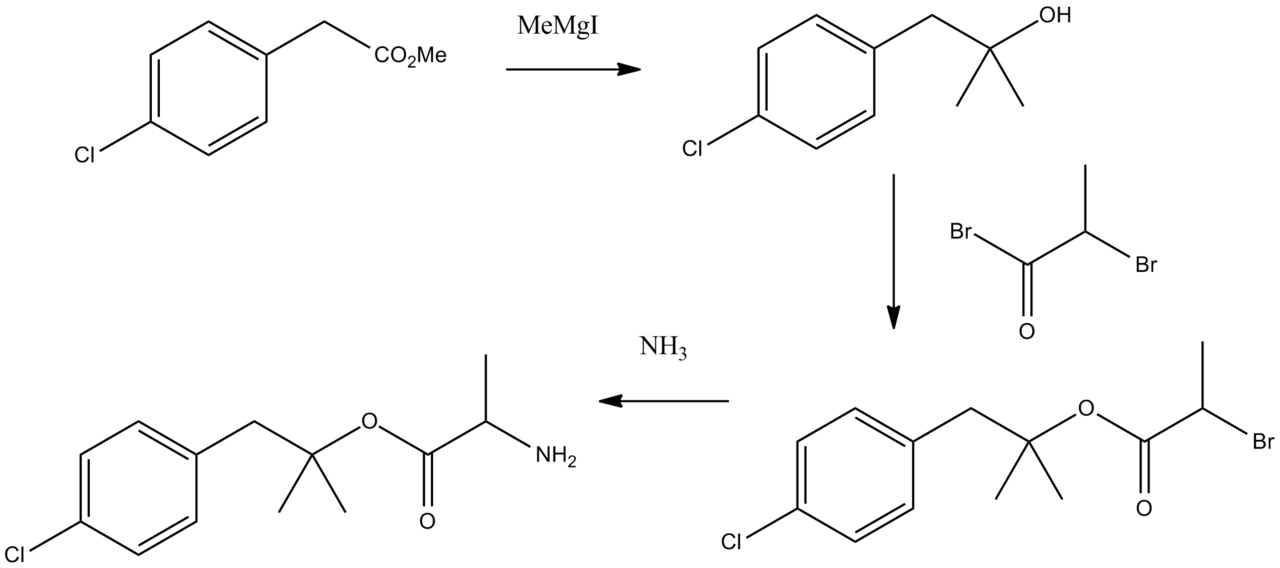
Synthesis
See also
References
- ↑ Wilkinson A, Courtney M, Westlind-Danielsson A, Hallnemo G, Akerman KE (December 1994). "Alaproclate acts as a potent, reversible and noncompetitive antagonist of the NMDA receptor coupled ion flow". The Journal of Pharmacology and Experimental Therapeutics. 271 (3): 1314–9. PMID 7996440. (Subscription required (help)).
- ↑ Nicholson KL, Balster RL (November 2003). "Evaluation of the phencyclidine-like discriminative stimulus effects of novel NMDA channel blockers in rats". Psychopharmacology (Berlin). 170 (2): 215–24. doi:10.1007/s00213-003-1527-6. PMID 12851738. (Subscription required (help)).
- ↑ Template:Cite doi
- Pages with script errors
- Pages containing links to subscription-only content
- CS1 maint: Multiple names: authors list
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Selective serotonin reuptake inhibitors
- Organochlorides
- Propionates
- NMDA receptor antagonists
- Drug