Agalsidase beta
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Agalsidase beta is a Endocrine-Metabolic Agent that is FDA approved for the treatment of Fabry disease. Common adverse reactions include infusion reactions.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Fabrazyme® (agalsidase beta) is indicated for use in patients with Fabry disease. Fabrazyme reduces globotriaosylceramide (GL‑3) deposition in capillary endothelium of the kidney and certain other cell types.
Recommended Dose The recommended dosage of Fabrazyme is 1 mg/kg body weight infused every two weeks as an intravenous (IV) infusion. Patients should receive antipyretics prior to infusion [see WARNINGS AND PRECAUTIONS (5.2)].
The initial IV infusion rate should be no more than 0.25 mg/min (15 mg/hr). The infusion rate may be slowed in the event of infusion reactions. After patient tolerance to the infusion is well established, the infusion rate may be increased in increments of 0.05 to 0.08 mg/min (increments of 3 to 5 mg/hr) with each subsequent infusion. For patients weighing < 30 kg, the maximum infusion rate should remain at 0.25 mg/min (15 mg/hr). For patients weighing ≥ 30 kg, the administration duration should not be less than 1.5 hours (based on individual patient tolerability).
Patients who have had a positive skin test to Fabrazyme or who have tested positive for anti-Fabrazyme IgE may be successfully re-challenged with Fabrazyme. The initial re-challenge administration should be a low dose at a lower infusion rate, e.g., 1/2 the therapeutic dose (0.5 mg/kg) at 1/25 the initial standard recommended rate (0.01 mg/min). Once a patient tolerates the infusion, the dose may be increased to reach the approved dose of 1 mg/kg and the infusion rate may be increased by slowly titrating upwards (doubled every 30 minutes up to a maximum rate of 0.25 mg/min), as tolerated.
3 DOSAGE FORMS AND STRENGTHS Fabrazyme is supplied as a sterile, nonpyrogenic, white to off-white, lyophilized cake or powder for reconstitution with Sterile Water for Injection, USP to yield a concentration of 5 mg/mL; and then further diluted with 0.9% Sodium Chloride Injection, USP for intravenous infusion.
Single-use vials are available in 35 mg and 5 mg dosages.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Agalsidase beta in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Agalsidase beta in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Agalsidase beta in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Agalsidase beta in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Agalsidase beta in pediatric patients.
Contraindications
- None
Warnings
Anaphylaxis and Allergic Reactions Life-threatening anaphylactic and severe allergic reactions have been observed in patients during Fabrazyme infusions. Reactions have included localized angioedema (including swelling of the face, mouth, and throat), bronchospasm, hypotension, generalized urticaria, dysphagia, rash, dyspnea, flushing, chest discomfort, pruritus, and nasal congestion. Interventions have included cardiopulmonary resuscitation, oxygen supplementation, IV fluids, hospitalization, and treatment with inhaled beta-adrenergic agonists, epinephrine, and IV corticosteroids.
In clinical trials and postmarketing safety experience with Fabrazyme, approximately 1% of patients developed anaphylactic or severe allergic reactions during Fabrazyme infusion.
If anaphylactic or severe allergic reactions occur, immediately discontinue the administration of Fabrazyme and initiate necessary emergency treatment. Because of the potential for severe allergic reactions, appropriate medical support measures should be readily available when Fabrazyme is administered.
The risks and benefits of re-administering Fabrazyme following an anaphylactic or severe allergic reaction should be considered. Extreme care should be exercised, with appropriate medical support measures readily available, if the decision is made to re-administer the product [see WARNINGS AND PRECAUTIONS (5.4) and CLINICAL STUDIES (14)].
5.2 Infusion Reactions In clinical trials with Fabrazyme, approximately 50-55% of patients experienced infusion reactions during Fabrazyme administration, some of which were severe [see WARNINGS AND PRECAUTIONS (5.1)]. Severe infusion reactions experienced by more than one patient in clinical studies with Fabrazyme included chills, vomiting, hypotension, and paresthesia. Other infusion reactions included pyrexia, feeling hot or cold, dyspnea, nausea, flushing, headache, fatigue, pruritus, pain in extremity, hypertension, chest pain, throat tightness, abdominal pain, dizziness, tachycardia, nasal congestion, diarrhea, edema peripheral, myalgia, urticaria, bradycardia, and somnolence.
Most patients in clinical trials were pretreated with acetaminophen. In patients experiencing infusion reactions, pretreatment with an antipyretic and antihistamine is recommended. Infusion reactions occurred in some patients after receiving pretreatment with antipyretics, antihistamines, and oral steroids. Infusion reactions tended to decline in frequency with continued use of Fabrazyme. However, infusion reactions may still occur despite extended duration of Fabrazyme treatment. If an infusion reaction occurs, decreasing the infusion rate, temporarily stopping the infusion, and/or administrating additional antipyretics, antihistamines, and/or steroids may ameliorate the symptoms. If severe infusion reactions occur, immediate discontinuation of the administration of Fabrazyme should be considered, and appropriate medical treatment should be initiated. Severe reactions are generally managed with administration of antihistamines, corticosteroids, intravenous fluids, and/or oxygen, when clinically indicated. Because of the potential for severe infusion reactions, appropriate medical support measures should be readily available when Fabrazyme is administered. Patients who have experienced infusion reactions should be treated with caution when re-administering Fabrazyme.
5.3 Compromised Cardiac Function Patients with advanced Fabry disease may have compromised cardiac function, which may predispose them to a higher risk of severe complications from infusion reactions [see WARNINGS AND PRECAUTIONS (5.1) and (5.2)]. Patients with compromised cardiac function should be monitored closely if the decision is made to administer Fabrazyme.
5.4 Immunogenicity and Re-challenge In clinical trials with Fabrazyme, a few patients developed IgE antibodies or skin test reactivity specific to Fabrazyme. Two of six patients in the re-challenge study discontinued treatment with Fabrazyme prematurely due to recurrent infusion reactions. Four serious infusion reactions occurred in three patients during Fabrazyme infusions, including bronchospasm, urticaria, hypotension, and development of Fabrazyme-specific antibodies. Other infusion-related reactions occurring in more than one patient during the study included rigors, hypertension, nausea, vomiting, and pruritus. Physicians should consider testing for IgE antibodies in patients who experienced suspected allergic reactions and consider the risks and benefits of continued treatment in patients with anti-Fabrazyme IgE antibodies [see WARNINGS AND PRECAUTIONS (5.1) and DOSAGE AND ADMINISTRATION (2)].
Patients who have had a positive skin test to Fabrazyme or who have tested positive for Fabrazyme-specific IgE antibody have been re-challenged with Fabrazyme using a re-challenge protocol [see CLINICAL STUDIES (14)]. Re-challenge of these patients should only occur under the direct supervision of qualified personnel, with appropriate medical support measures readily available.
5.5 Monitoring: Laboratory Tests There are no marketed tests for antibodies against Fabrazyme. If testing is warranted, contact your local Genzyme representative or Genzyme Corporation at (800) 745-4447.
Adverse Reactions
Clinical Trials Experience
Adverse Reactions in Clinical Studies Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in patients in clinical practice.
The most serious adverse reactions reported with Fabrazyme treatment during clinical trials were anaphylactic and allergic reactions [see WARNINGS AND PRECAUTIONS (5.1)].
The most common adverse reactions reported with Fabrazyme are infusion reactions, some of which were severe [see WARNINGS AND PRECAUTIONS (5.1) and (5.2)]. Serious and/or frequently occurring (≥ 5% incidence) related adverse reactions consisted of one or more of the following: chills, pyrexia, feeling hot or cold, dyspnea, nausea, flushing, headache, vomiting, paresthesia, fatigue, pruritus, pain in extremity, hypertension, chest pain, throat tightness, abdominal pain, dizziness, tachycardia, nasal congestion, diarrhea, edema peripheral, myalgia, back pain, pallor, bradycardia, urticaria, hypotension, face edema, rash, and somnolence. The occurrence of somnolence can be attributed to clinical trial specified pretreatment with antihistamines. Most infusion-related reactions requiring intervention were ameliorated with slowing of the infusion rate, temporarily stopping the infusion, and/or administration of antipyretics, antihistamines, or steroids.
Other reported serious adverse events included stroke, pain, ataxia, bradycardia, cardiac arrhythmia, cardiac arrest, decreased cardiac output, vertigo, hypoacousia, and nephrotic syndrome. These adverse events also occur as manifestations of Fabry disease; an alteration in frequency or severity cannot be determined from the small numbers of patients studied.
The data described below reflect exposure of 80 patients, ages 16 to 61 years, to 1 mg/kg Fabrazyme every two weeks in two separate double-blind, placebo-controlled clinical trials, for periods ranging from 1 to 35 months (mean 15.5 months). All 58 patients enrolled in one of the two studies continued into an open-label extension study of Fabrazyme treatment for up to 54 additional months. Patients were treated with antipyretics and antihistamines prior to the infusions.
TABLE 2 enumerates treatment-emergent adverse reactions (regardless of relationship) that occurred during the double-blind treatment periods of the two placebo-controlled trials (Study 1 and Study 2) [see CLINICAL STUDIES (14)]. Reported adverse reactions have been classified by Medical Dictionary for Regulatory Activities (MedDRA) terminology System Organ Class and Preferred Term.
Observed adverse reactions in the Phase 1/2 study and the open-label treatment period for the extension study following the controlled study were not different in nature or intensity.
The safety profile of Fabrazyme in pediatric Fabry disease patients, ages 8 to 16 years, was found to be consistent with that seen in adults [see USE IN SPECIFIC POPULATIONS (8.4) and CLINICAL STUDIES (14)]. The safety of Fabrazyme in patients younger than 8 years of age has not been evaluated.
6.2 Immunogenicity Ninety-five of 121 (79%) adult patients and 11 of 16 (69%) pediatric patients (106 of 137, 74% of all patients) treated with Fabrazyme in clinical studies have developed IgG antibodies to Fabrazyme. Most patients who develop IgG antibodies do so within the first three months of exposure. IgG seroconversion in pediatric patients was associated with prolonged half-life of Fabrazyme, a phenomenon rarely observed in adult patients [see CLINICAL PHARMACOLOGY (12.3) and USE IN SPECIFIC POPULATIONS (8.4)]. A possible cause for this prolongation likely pertains to the ability of antibodies to act as “carriers” for their antigens. Among the 14 female patients exposed to Fabrazyme in clinical studies, six (adult patients) developed IgG antibodies to Fabrazyme.
IgG antibodies to Fabrazyme were purified from 15 patients with high antibody titers (≥ 12,800) and studied for inhibition of in vitro enzyme activity. Under the conditions of this assay, most of these 15 patients had inhibition of in vitro enzyme activity ranging between 21-74% at one or more time points during the study. Assessment of inhibition of enzyme uptake in cells has not been performed. No general pattern was seen in individual patient reactivity over time. The clinical significance of binding and/or inhibitory antibodies to Fabrazyme is not known. In patients followed in the open-label extension study, reduction of GL-3 in plasma and GL-3 inclusions in superficial skin capillaries was maintained after antibody formation.
As with all therapeutic proteins, there is potential for immunogenicity. The data reflect the percentage of patients whose test results were considered positive for antibodies to Fabrazyme using an ELISA and radioimmunoprecipitation (RIP) assay for antibodies. The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Fabrazyme with the incidence of antibodies to other products may be misleading.
Testing for IgE antibodies was performed in approximately 60 patients in clinical trials who experienced moderate to severe infusion reactions or in whom mast cell activation was suspected. Seven of these patients tested positive for Fabrazyme-specific IgE antibodies or had a positive skin test to Fabrazyme. Patients who have had a positive skin test to Fabrazyme, or who have tested positive for Fabrazyme-specific IgE antibodies in clinical trials with Fabrazyme have been re-challenged
Postmarketing Experience
The following adverse reactions have been identified during post approval use of FABRAZYME. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In postmarketing experience with agalsidase beta, severe and serious infusion-related reactions have been reported, some of which were life-threatening, including anaphylactic shock [see WARNINGS AND PRECAUTIONS (5.1)]. Reactions have included localized angioedema (including auricular swelling, eye swelling, dysphagia, lip swelling, edema, pharyngeal edema, face swelling, and swollen tongue), generalized urticaria, bronchospasm, and hypotension.
Adverse reactions (regardless of relationship) resulting in death reported in the postmarketing setting with FABRAZYME treatment included cardiorespiratory arrest, respiratory failure, cardiac failure, sepsis, cerebrovascular accident, myocardial infarction, renal failure, and pneumonia. Some of these reactions were reported in Fabry disease patients with significant underlying disease.
In addition to the adverse reactions reported in ADVERSE REACTIONS IN CLINICAL STUDIES (6.1), the following adverse reactions have been reported during postmarketing use of agalsidase beta: arthralgia, asthenia, erythema, hyperhidrosis, infusion site reaction, lacrimation increased, leukocytoclastic vasculitis, lymphadenopathy, hypoesthesia, oral hypoesthesia, palpitations, rhinorrhea, oxygen saturation decreased, and hypoxia.
Drug Interactions
Interference with Other Drugs No drug interaction studies were performed.
No in vitro metabolism studies were performed.
7.2 Interference with Laboratory Tests There is no known interference by Fabrazyme with laboratory tests. Antibody samples should be collected prior to Fabrazyme infusions.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of Fabrazyme use in pregnant women. Reproduction studies performed in rats at doses up to 30 times the human dose have revealed no evidence of impaired fertility or negative effects on embryo fetal development due to Fabrazyme. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Women of childbearing potential should be encouraged to enroll in the Fabry patient registry. The registry will monitor the effect of Fabrazyme on pregnant women and their offspring. For more information, visit www.fabryregistry.com or call (800) 745-4447
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Agalsidase beta in women who are pregnant.
Labor and Delivery
There is no information on the effect of Fabrazyme during labor and delivery. Pregnant females are encouraged to enroll in the Fabry registry
Nursing Mothers
- It is not known whether Fabrazyme is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Fabrazyme is administered to a nursing woman.
Nursing mothers should be encouraged to enroll in the Fabry registry
Pediatric Use
- The safety and efficacy of Fabrazyme were assessed in a multi-national, multi-center, uncontrolled, open-label study in 16 pediatric patients with Fabry disease (14 males, 2 females), ages 8 to 16 years [see CLINICAL STUDIES (14)]. Patients younger than 8 years of age were not included in clinical studies. The safety and efficacy in patients younger than 8 years of age have not been evaluated.
Geriatic Use
Clinical studies of Fabrazyme did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
Fabry disease is an X-linked genetic disorder. However, some heterozygous women will develop signs and symptoms of Fabry disease due to the variability of the X-chromosome inactivation within cells.
A total of 12 adult female patients with Fabry disease were enrolled in two separate randomized, double-blind, placebo-controlled clinical studies with Fabrazyme, and two female pediatric patients with Fabry disease, ages 11 years, were evaluated in an open-label, uncontrolled pediatric study [see USE IN SPECIFIC POPULATIONS (8.4) and CLINICAL STUDIES (14)]. Although the safety and efficacy data available in female patients in these clinical studies are limited, there is no indication that female patients respond differently to Fabrazyme compared to males.
Race
There is no FDA guidance on the use of Agalsidase beta with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Agalsidase beta in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Agalsidase beta in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Agalsidase beta in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Agalsidase beta in patients who are immunocompromised.
Administration and Monitoring
Administration
Instructions for Use Fabrazyme does not contain any preservatives. Vials are for single use only. Discard any unused product.
Avoid shaking or agitating this product. Do not use filter needles during the preparation of the infusion.
Reconstitution and Dilution (using Aseptic Technique)
Allow Fabrazyme vials and diluent to reach room temperature prior to reconstitution (approximately 30 minutes). The number of 35 mg and 5 mg vials needed is based on the patient’s body weight (kg) and the recommended dose of 1 mg/kg.
Select a combination of 35 mg and 5 mg vials so that the total number of mg is equal to or greater than the patient’s number of kg of body weight.
Reconstitute each 35 mg vial of Fabrazyme by slowly injecting 7.2 mL of Sterile Water for Injection, USP down the inside wall of each vial. Roll and tilt each vial gently. Each vial will yield a 5 mg/mL clear, colorless solution (total extractable amount per vial is 35 mg, 7 mL).
Reconstitute each 5 mg vial of Fabrazyme by slowly injecting 1.1 mL of Sterile Water for Injection, USP down the inside wall of each vial. Roll and tilt each vial gently. Each vial will yield a 5 mg/mL clear, colorless solution (total extractable amount per vial is 5 mg, 1 mL).
Visually inspect the reconstituted vials for particulate matter and discoloration. Do not use the reconstituted solution if there is particulate matter or if it is discolored.
The reconstituted solution should be further diluted with 0.9% Sodium Chloride Injection, USP to a total volume based on patient weight specified in TABLE 1 below. Prior to adding the volume of reconstituted Fabrazyme required for the patient dose, remove an equal volume of 0.9% Sodium Chloride Injection, USP from the infusion bag.
Patient dose (in mg) ÷ 5 mg/mL = Number of mL of reconstituted Fabrazyme required for patient dose
Example: Patient dose = 80 mg
80 mg ÷ 5 mg/mL = 16 mL of Fabrazyme
Slowly withdraw the reconstituted solution from each vial up to the total volume required for the patient dose. Inject the reconstituted Fabrazyme solution directly into the Sodium Chloride solution. Do not inject in the airspace within the infusion bag. Discard any vial with unused reconstituted solution.
Gently invert infusion bag to mix the solution, avoiding vigorous shaking and agitation.
Do not infuse Fabrazyme in the same intravenous line with other products.
Administer FABRAZYME using an in-line low protein binding 0.2 µm filter.
Monitoring
- Patients with compromised cardiac function should be monitored closely if the decision is made to administer Fabrazyme.
IV Compatibility
There is limited information regarding IV Compatibility of Agalsidase beta in the drug label.
Overdosage
There have been no reports of overdose with Fabrazyme. In clinical trials, patients received doses up to 3 mg/kg body weight. The adverse reactions experienced by patients who received treatment with 3 mg/kg were similar to the adverse reactions experienced by patients who received treatment with 1 mg/kg.
Pharmacology
There is limited information regarding Agalsidase beta Pharmacology in the drug label.
Mechanism of Action
- Fabry disease is an X-linked genetic disorder of glycosphingolipid metabolism. Deficiency of the lysosomal enzyme α-galactosidase A leads to progressive accumulation of glycosphingolipids, predominantly GL-3, in many body tissues, starting early in life and continuing over decades. Clinical manifestations of Fabry disease include renal failure, cardiomyopathy, and cerebrovascular accidents. Accumulation of GL-3 in renal endothelial cells may play a role in renal failure.
Fabrazyme is intended to provide an exogenous source of α‑galactosidase A in Fabry disease patients. Nonclinical and clinical studies evaluating a limited number of cell types indicate that Fabrazyme will catalyze the hydrolysis of glycosphingolipids, including GL-3.
Structure
- Fabrazyme (agalsidase beta) is a recombinant human α-galactosidase A enzyme with the same amino acid sequence as the native enzyme. Purified agalsidase beta is a homodimeric glycoprotein with a molecular weight of approximately 100 kD. The mature protein is comprised of two subunits of 398 amino acids (approximately 51 kD), each of which contains three N-linked glycosylation sites. α‑galactosidase A catalyzes the hydrolysis of globotriaosylceramide (GL-3) and other α‑galactyl-terminated neutral glycosphingolipids, such as galabiosylceramide and blood group B substances to ceramide dihexoside and galactose. The specific activity of Fabrazyme is approximately 70 U/mg (one unit is defined as the amount of activity that results in the hydrolysis of 1 µmole of a synthetic substrate, p-nitrophenyl-α-D-galactopyranoside, per minute under the assay conditions).
Fabrazyme is produced by recombinant DNA technology in a Chinese Hamster Ovary mammalian cell expression system.
Fabrazyme is intended for intravenous infusion. It is supplied as a sterile, nonpyrogenic, white to off-white, lyophilized cake or powder for reconstitution with Sterile Water for Injection, USP. Each 35 mg vial contains 37 mg of agalsidase beta, as well as 222 mg mannitol, 20.4 mg sodium phosphate monobasic monohydrate, and 59.2 mg sodium phosphate dibasic heptahydrate. Following reconstitution as directed, 35 mg of agalsidase beta (7 mL) may be extracted from each 35 mg vial.
Each 5 mg vial contains 5.5 mg of agalsidase beta, as well as 33.0 mg mannitol, 3.0 mg sodium phosphate monobasic monohydrate, and 8.8 mg sodium phosphate dibasic heptahydrate. Following reconstitution as directed, 5 mg of agalsidase beta (1 mL) may be extracted from each 5 mg vial.
Pharmacodynamics
In a placebo-controlled study conducted in patients with Fabry disease after intravenous administration of 1 mg/kg of Fabrazyme every two weeks for 20 weeks, a reduction of GL-3 was observed in the capillary endothelium (vasculature) of kidney, heart and skin as determined by histological assessment, and in plasma as determined by ELISA
Pharmacokinetics
Plasma pharmacokinetic profiles of Fabrazyme were characterized at 0.3, 1, and 3 mg/kg in adult patients with Fabry disease. The area under the plasma concentration-time curve (AUC∞) and the clearance (CL) did not increase proportionately with increasing doses, demonstrating that the enzyme follows non-linear pharmacokinetics (TABLE 3). Plasma pharmacokinetic profiles were also characterized in adult patients with Fabry disease given 1 mg/kg Fabrazyme every 14 days for a total of 11 infusions. Refer to TABLE 3 below for more details.
In 15 pediatric Fabry patients (ranging in age from 8 to 16 years old and weighing between 27.1 to 64.9 kg) who were dosed with 1 mg/kg every 14 days, Fabrazyme pharmacokinetics were not weight-dependent (TABLE 3). Fabrazyme concentrations were about five times higher after IgG seroconversion, without any detectable impact on GL-3 clearance.
IgG seroconversion in pediatric patients was associated with prolonged half-life and plasma concentrations of Fabrazyme, a phenomenon rarely observed in adult patients. A possible cause for this prolongation likely pertains to the ability of antibodies to potentially act as “carriers” for their antigens
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility There are no animal or human studies to assess the carcinogenic or mutagenic potential of Fabrazyme. There are no studies assessing the potential effect of Fabrazyme on fertility in humans.
Clinical Studies
The safety and efficacy of Fabrazyme were assessed in four clinical studies in patients with Fabry disease.
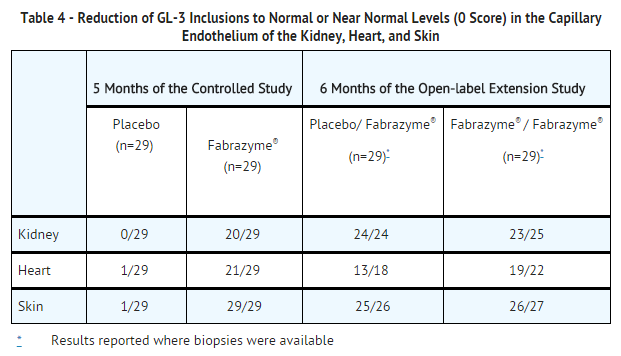
Study 1 was a randomized, double-blind, placebo-controlled, multi-national, multi-center study of 58 Fabry patients (56 males and 2 females), ages 16 to 61 years, all naïve to enzyme replacement therapy. Patients received either 1 mg/kg of Fabrazyme or placebo every two weeks for five months (20 weeks) for a total of 11 infusions. All patients were pretreated with acetaminophen and an antihistamine to decrease or prevent infusion reactions. Oral steroids were an additional option to the pretreatment regimen for patients who exhibited severe or recurrent infusion reactions. The primary efficacy endpoint of GL-3 inclusions in renal interstitial capillary endothelial cells, was assessed by light microscopy and was graded on an inclusion severity score ranging from 0 (normal or near normal) to 3 (severe inclusions).
A GL-3 inclusion score of 0 was achieved in 20 of 29 (69%) patients treated with Fabrazyme compared to 0 of 29 treated with placebo (p<0.001). Similar reductions in GL-3 inclusions were observed in the capillary endothelium of the heart and skin (TABLE 4). No differences between groups in symptoms or renal function were observed during this five-month study.
All 58 patients in Study 1 participated in an open-label extension study of Fabrazyme at 1 mg/kg every two weeks, which continued for an additional 54 months. At the end of six months of open-label treatment, most patients achieved a GL-3 inclusion score of 0 in capillary endothelium (TABLE 4). GL-3 was decreased to normal or near normal levels in mesangial cells, glomerular capillary endothelium, interstitial cells, and non-capillary endothelium. GL-3 deposition was still present in vascular smooth muscle cells, tubular epithelium and podocytes, at variably reduced levels. Forty-four of the 58 patients completed 54 months of the open-label extension study. Thirty-six of these 44 patients underwent follow-up skin biopsy, and 31 of these patients showed sustained GL-3 clearance in the capillary endothelium of the skin. Follow-up heart and kidney biopsies were assessed in only 8 of the 44 patients, which showed sustained GL-3 clearance in the capillary endothelium of the kidney in 8 patients, and sustained GL-3 clearance in the capillary endothelium of the heart in 6 patients. Plasma GL-3 levels were reduced to normal levels (≤ 7.03 µg/mL determined by LC/MS/MS) and remained at normal levels after up to 60 months of treatment. The reduction of GL-3 inclusions suggests that Fabrazyme may ameliorate disease expression; however, the relationship of GL-3 inclusion reduction to specific clinical manifestations of Fabry disease has not been established.
Study 2 was a randomized (2:1 Fabrazyme to placebo), double-blind, placebo-controlled, multi-national, multi-center study of 82 patients (72 males and 10 females), ages 20 to 72 years, all naïve to enzyme replacement therapy. Patients received either 1 mg/kg of Fabrazyme or placebo every two weeks for up to a maximum of 35 months (median 18.5 months). There was significant difference in post-baseline plasma GL-3 levels in the Fabrazyme-treated patients compared to placebo. The reduction in plasma GL-3 levels in the Fabrazyme group compared to the placebo group was significant at one year (p<0.0001) and at two years (p=0.0019). Fourteen patients (8 in Fabrazyme-treated and 6 in placebo) had skin biopsies at first infusion and final visit. All Fabrazyme-treated patients had capillary endothelium and deep vessel endothelium scores of zero at the final visit. Four (4) of 6 placebo patients had non-zero capillary endothelium scores (p=0.0150), and 6 of 6 had non-zero deep vessel endothelium scores (p=0.0003).
Sixty-seven patients who participated in Study 2 were subsequently entered into an open-label extension study in which all patients received 1 mg/kg of Fabrazyme every two weeks for up to a maximum of 18 months. There was a statistically significant reduction in mean plasma GL-3 levels with durability in effect through the additional 18 months of treatment in the extension study from pretreatment baseline.
Study 3 (Pediatric Study) was an open-label, uncontrolled, multi-national, multi-center study to evaluate safety, pharmacokinetics, and pharmacodynamics of Fabrazyme treatment in 16 pediatric patients with Fabry disease (14 males, 2 females), who were ages 8 to 16 years at first treatment. All patients received Fabrazyme 1 mg/kg every two weeks for up to 48 weeks. At baseline, all 14 males had elevated plasma GL-3 levels (i.e., > 7.03 µg/mL), whereas the two female patients had normal plasma GL-3 levels. Twelve of the 14 male patients, and no female patients, had GL-3 inclusions observed in the capillary endothelium on skin biopsies at baseline. At Weeks 24 and 48 of treatment, all 14 males had plasma GL-3 within the normal range. The 12 male patients with GL-3 inclusions in capillary endothelium at baseline achieved GL-3 inclusion scores of 0 at Weeks 24 and 48 of treatment. The two female patients’ plasma GL-3 levels remained normal through study Week 48.
No new safety concerns were identified in pediatric patients in this study, and the overall safety and efficacy profile of Fabrazyme treatment in pediatric patients was found to be consistent with that seen in adults. Immunologic responses in pediatric patients may differ from those in adults, as IgG seroconversion in pediatric patients was associated with prolonged half-life concentrations of Fabrazyme, a phenomenon rarely observed in adult patients [see CLINICAL PHARMACOLOGY (12.3), ADVERSE REACTIONS (6.2), and USE IN SPECIFIC POPULATIONS (8.4)].
Study 4 was an open-label, re-challenge study to evaluate the safety of Fabrazyme treatment in patients who had a positive skin test to Fabrazyme or who had tested positive for Fabrazyme-specific IgE antibodies. In this study, six adult male patients, who had experienced multiple or recurrent infusion reactions during previous clinical trials with Fabrazyme, were re-challenged with Fabrazyme administered as a graded infusion, for up to 52 weeks of treatment [see WARNINGS AND PRECAUTIONS (5.4)]. The initial two re-challenge doses of Fabrazyme were administered as a 0.5 mg/kg dose per week at an initial infusion rate of 0.01 mg/min for the first 30 minutes (1/25th the usually recommended maximum infusion rate). The infusion rate was doubled every 30 minutes thereafter, as tolerated, for the remainder of the infusion up to a maximum rate of 0.25 mg/min. If the patient tolerated the infusion, the dose was increased to 1 mg/kg every two weeks (usually recommended dose), and the infusion rate was increased by slow titration upwards [see DOSAGE AND ADMINISTRATION (2)]. Four of the six patients treated in this study received at least 26 weeks of study medication, and two patients discontinued prematurely due to recurrent infusion reactions
How Supplied
- Fabrazyme is supplied as a sterile, nonpyrogenic, white to off-white lyophilized cake or powder. Fabrazyme 35 mg vials are supplied in single-use, clear Type I glass 20 mL (cc) vials. The closure consists of a siliconized butyl stopper and an aluminum seal with a plastic purple flip-off cap. Fabrazyme 5 mg vials are supplied in single-use, clear Type I glass 5 mL (cc) vials. The closure consists of a siliconized butyl stopper and an aluminum seal with a plastic gray flip-off cap.
35 mg vial: NDC 58468-0040-1
5 mg vial: NDC 58468-0041-1
Storage
- Refrigerate vials of Fabrazyme at 2° to 8°C (36° to 46°F). DO NOT USE Fabrazyme after the expiration date on the vial. Reconstituted and diluted solutions of Fabrazyme should be used immediately. This product contains no preservatives. If immediate use is not possible, the reconstituted and diluted solution may be stored for up to 24 hours at 2° to 8°C (36° to 46°F).
Images
Drug Images
{{#ask: Page Name::Agalsidase beta |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL

This image is provided by the National Library of Medicine. 
This image is provided by the National Library of Medicine.
Ingredients and Appearance
{{#ask: Label Page::Agalsidase beta |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be informed that a Registry has been established in order to better understand the variability and progression of Fabry disease in the population as a whole and in women [see USE IN SPECIFIC POPULATIONS (8.6)], and to monitor and evaluate long-term treatment effects of Fabrazyme. The Registry will also monitor the effect of Fabrazyme on pregnant women and their offspring. Patients should be encouraged to participate and advised that their participation is voluntary and may involve long-term follow-up. For more information, visit www.fabryregistry.com or call (800) 745-4447.
Precautions with Alcohol
- Alcohol-Agalsidase beta interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Fabrazyme®[1]
Look-Alike Drug Names
There is limited information regarding Agalsidase beta Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Agalsidase beta
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Agalsidase beta |Label Name=Agalsidase beta11.png
}}
{{#subobject:
|Label Page=Agalsidase beta |Label Name=Agalsidase beta11.png
}}