Afatinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Afatinib is a kinase inhibitor that is FDA approved for the treatment of metastatic non-small cell lung cancer (NSCLC). Common adverse reactions include diarrhea, rash/dermatitis acneiform, stomatitis, paronychia, dry skin, decreased appetite, pruritus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Metastatic Non-Small Cell Lung Cancer
- Dosing Information
- The recommended dose of Afatinib is 40 mg orally once daily until disease progression or no longer tolerated by the patient. Take Afatinib at least 1 hour before or 2 hours after a meal.
- Do not take a missed dose within 12 hours of the next dose.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Afatinib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Afatinib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Afatinib in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Afatinib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Afatinib in pediatric patients.
Contraindications
- None
Warnings
Precautions
- Diarrhea
- Diarrhea has resulted in dehydration with or without renal impairment; some of these cases were fatal. In Study 1, diarrhea occurred in 96% of patients treated with Afatinib (n=229), of which 15% was Grade 3 in severity and occurred within the first 6 weeks. Renal impairment as a consequence of diarrhea occurred in 6.1% of patients treated with Afatinib, out of which 3 (1.3%) were Grade 3.
- For patients who develop prolonged Grade 2 diarrhea lasting more than 48 hours or greater than or equal to Grade 3 diarrhea, withhold Afatinib until diarrhea resolves to Grade 1 or less, and resume Afatinib with appropriate dose reduction. Provide patients with an anti-diarrheal agent (e.g., loperamide) for self-administration at the onset of diarrhea and instruct patients to continue anti-diarrheal therapy until loose bowel movements cease for 12 hours.
- Bullous and Exfoliative Skin Disorders
- Grade 3 cutaneous reactions characterized by bullous, blistering, and exfoliating lesions occurred in 6 (0.15%) of the 3865 patients who received Afatinib across clinical trials. In Study 1, the overall incidence of cutaneous reactions consisting of rash, erythema, and acneiform rash was 90%, and the incidence of Grade 3 cutaneous reactions was 16%. In addition, the incidence of Grade 1-3 palmar-plantar erythrodysesthesia syndrome was 7%. Discontinue Afatinib in patients who develop life-threatening bullous, blistering, or exfoliating lesions. For patients who develop prolonged Grade 2 cutaneous adverse reactions lasting more than 7 days, intolerable Grade 2, or Grade 3 cutaneous reactions, withhold Afatinib until the adverse reaction resolves to Grade 1 or less, and resume Afatinib with appropriate dose reduction.
- Interstitial Lung Disease (ILD)
- ILD or ILD-like adverse reactions (e.g., lung infiltration, pneumonitis, acute respiratory distress syndrome, or alveolitis allergic) occurred in 1.5% of the 3865 patients who received Afatinib across clinical trials; of these, 0.4% were fatal. The incidence of ILD appeared to be higher in patients of Asian ethnicity (2.1%) as compared to non-Asians (1.2%). In Study 1, the incidence of Grade ≥3 ILD was 1.3% and resulted in death in 1% of Afatinib-treated patients.
- Withhold Afatinib during evaluation of patients with suspected ILD, and discontinue Afatinib in patients with confirmed ILD.
- Hepatic Toxicity
- In 3865 patients who received Afatinib across clinical trials, 10.1% had liver test abnormalities, of which 7 (0.18%) were fatal. In Study 1, liver test abnormalities of any grade occurred in 17.5% of the patients treated with Afatinib.
- Obtain periodic liver testing in patients during treatment with Afatinib. Withhold Afatinib in patients who develop worsening of liver function. In patients who develop severe hepatic impairment while taking Afatinib, treatment should be discontinued.
- Keratitis
- Keratitis, characterized as acute or worsening eye inflammation, lacrimation, light sensitivity, blurred vision, eye pain, and/or red eye occurred in 0.8% of patients treated with Afatinib among 3865 patients across clinical trials. Keratitis was reported in 5 (2.2%) patients in Study 1, with Grade 3 in 1 (0.4%). Withhold Afatinib during evaluation of patients with suspected keratitis, and if diagnosis of ulcerative keratitis is confirmed, treatment with Afatinib should be interrupted or discontinued. If keratitis is diagnosed, the benefits and risks of continuing treatment should be carefully considered. Afatinib should be used with caution in patients with a history of keratitis, ulcerative keratitis, or severe dry eye. Contact lens use is also a risk factor for keratitisand ulceration.
- Embryofetal Toxicity
- Based on its mechanism of action, Afatinib can cause fetal harm when administered to a pregnant woman. Afatinib was embryotoxic and, in animals with maternal toxicity, led to abortions at late gestational stages in rabbits at doses of 5 mg/kg (approximately 0.2 times the human exposure at the recommended dose of 40 mg daily) or greater. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Advise females of reproductive potential to use highly effective contraception during treatment, and for at least 2 weeks after the last dose of Afatinib. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking Afatinib.
- Combination with Vinorelbine in HER2 Positive Metastatic Breast Cancer
- An early interim overall survival analysis of a randomized Phase 3 trial in HER2 positive metastatic breast cancer showed an increased mortality in patients receiving Afatinib in combination with vinorelbine compared to trastuzumab and vinorelbine. The combination of Afatinib and vinorelbine was also associated with a higher rate of adverse events (such as diarrhea, rash) and fatal events related to infections and cancer progression. Afatinib combined with vinorelbine should not be used in patients with HER2 positive metastatic breast cancer.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety evaluation of Afatinib is based on the data from more than 3800 patients, including 2135 NSCLC patients receiving Afatinib monotherapy at or above the recommended dose.
- Controlled Study
- The data in Tables 1 and 2 below reflect exposure of 229 EGFR-TKI naïve Afatinib-treated patients with EGFR mutation-positive, metastatic, non-squamous, NSCLC enrolled in a randomized, multicenter, open-label trial (Study 1). Patients received Afatinib 40 mg daily until documented disease progression or intolerance to the therapy. A total of 111 patients were treated with pemetrexed/cisplatin. Patients were treated with pemetrexed 500 mg/m² followed after 30 minutes by cisplatin 75 mg/m² every three weeks for a maximum of six treatment courses.
- The median exposure was 11.0 months for patients treated with Afatinib and 3.4 months for patients treated with pemetrexed/cisplatin. The overall trial population had a median age of 61 years; 61% of patients in the Afatinib arm and 60% of patients in the pemetrexed/cisplatin arm were younger than 65 years. A total of 64% of patients on Afatinib and 67% of pemetrexed/cisplatin patients were female. More than two-thirds of patients were from Asia (Afatinib 70%; pemetrexed/cisplatin 72%).
- Serious adverse reactions were reported in 29% of patients treated with Afatinib. The most frequent serious adverse reactions reported in patients treated with Afatinib were diarrhea (6.6%); vomiting (4.8%); and dyspnea, fatigue, and hypokalemia (1.7% each). Fatal adverse reactions in Afatinib-treated patients in Study 1 included pulmonary toxicity/ILD-like adverse reactions (1.3%), sepsis (0.43%), and pneumonia (0.43%).
- Dose reductions due to adverse reactions were required in 57% of Afatinib-treated patients. The most frequent adverse reactions that led to dose reduction in the patients treated with Afatinib were diarrhea (20%), rash/acne (19%), paronychia (14%), and stomatitis (10%).
- Discontinuation of therapy in Afatinib-treated patients for adverse reactions was 14.0%. The most frequent adverse reactions that led to discontinuation in Afatinib-treated patients were diarrhea (1.3%), ILD (0.9%), and paronychia (0.9%).
- Clinical trials of Afatinib excluded patients with an abnormal left ventricular ejection fraction (LVEF), i.e., below the institutional lower limit of normal. In Study 1, all patients were evaluated for LVEF at screening and every 9 weeks thereafter in the Afatinib-treated group and as needed in the pemetrexed/cisplatin group. More Afatinib-treated patients (2.2%; n=5) experienced ventricular dysfunction (defined as diastolic dysfunction, left ventricular dysfunction, or ventricular dilation; all < Grade 3) compared to chemotherapy-treated patients (0.9%; n=1).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Afatinib in the drug label.
Drug Interactions
- Effect of P-glycoprotein (P-gp) Inhibitors and Inducers
- Oral administration of a P-gp inhibitor (ritonavir at 200 mg twice daily) 1 hour before administration of Afatinib increased systemic exposure to afatinib by 48%. There was no change in afatinib exposure when ritonavir was administered simultaneously with or 6 hours after Afatinib. Concomitant taking of P-gp inhibitors (including but not limited to ritonavir, cyclosporine A, ketoconazole, itraconazole, erythromycin, verapamil, quinidine, tacrolimus, nelfinavir, saquinavir, and amiodarone) with Afatinib can increase exposure to afatinib.
- Co-administration with oral dose of a P-gp inducer (rifampicin at 600 mg once daily for 7 days) decreased exposure to afatinib by 34%. Concomitant taking of P-gp inducers (including but not limited to rifampicin, carbamazepine, phenytoin, phenobarbital, and St. John’s Wort) with Afatinib can decrease exposure to afatinib.
Use in Specific Populations
Pregnancy
- Pregnancy Category D
- Risk Summary
- Based on its mechanism of action, Afatinib can cause fetal harm when administered to a pregnant woman. Afatinib was embryotoxic and, in animals with maternal toxicity, led to abortions at late gestational stages in rabbits at doses of 5 mg/kg (approximately 0.2 times the exposure by AUC at the recommended human dose of 40 mg daily) or greater. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Animal Data
- Administration of afatinib to pregnant rabbits at doses of 5 mg/kg (approximately 0.2 times the exposure by AUC at the recommended human dose of 40 mg daily) or greater during the period of organogenesis caused increased post implantation loss and, in animals showing maternal toxicity, abortion at late gestational stages. In the same study, at the high dose level of 10 mg/kg (approximately 0.7 times the exposure by AUC at the recommended human dose of 40 mg daily) there were reduced fetal weights, and increases in the incidence of runts, as well as visceral and dermal variations. In an embryofetal development study in rats, there were skeletal alterations consisting of incomplete or delayed ossifications and reduced fetal weight at a dose of 16 mg/kg (approximately twice the exposure at the recommended human dose of 40 mg daily).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Afatinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Afatinib during labor and delivery.
Nursing Mothers
- It is not known whether afatinib is present in human milk. Afatinib was present in the milk of lactating rats at concentrations 80-150 times higher than those found in plasma from 1 to 6 hours after administration. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants from Afatinib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness of Afatinib in pediatric patients have not been established.
Geriatic Use
- Of the 3865 patients in the clinical studies of Afatinib, 32% of patients were 65 years and older, while 7% were 75 years and older. No overall differences in safety were observed between patients 65 years and over and younger patients. In Study 1, 39% of the 345 patients were 65 years of age or older and 4% were 75 years or older. No overall differences in effectiveness were observed between patients 65 years and older and younger patients.
Gender
There is no FDA guidance on the use of Afatinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Afatinib with respect to specific racial populations.
Renal Impairment
- Afatinib has not been studied in patients with severely impaired renal function (creatinine clearance [CLcr] <30 mL/min). Adjustments to the starting dose of Afatinib are not considered necessary in patients with mild (CLcr 60-89 mL/min) renal impairment. Closely monitor patients with moderate (CLcr 30-59 mL/min) to severe (CLcr <30 mL/min) renal impairment and adjust Afatinib dose if not tolerated.
Hepatic Impairment
- Afatinib has not been studied in patients with severe (Child Pugh C) hepatic impairment. Adjustments to the starting dose of Afatinib are not considered necessary in patients with mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment. Closely monitor patients with severe hepatic impairment and adjust Afatinib dose if not tolerated.
Females of Reproductive Potential and Males
- Females
- Counsel patients on pregnancy planning and prevention. Advise female patients of reproductive potential to use highly effective contraception during treatment with Afatinib, and for at least 2 weeks after the last dose of Afatinib. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking Afatinib.
Immunocompromised Patients
There is no FDA guidance one the use of Afatinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Afatinib in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Afatinib in the drug label.
Overdosage
Acute Overdose
- Overdose was reported in 2 healthy adolescents each of whom ingested 360 mg of Afatinib (as part of a mixed-drug ingestion) resulting in nausea, vomiting, asthenia, dizziness, headache, abdominal pain, and elevated amylase (<1.5 times upper limit of normal [ULN]). Both subjects recovered.
Chronic Overdose
There is limited information regarding Chronic Overdose of Afatinib in the drug label.
Pharmacology
Mechanism of Action
- Afatinib covalently binds to the kinase domains of EGFR (ErbB1), HER2 (ErbB2), and HER4 (ErbB4) and irreversibly inhibits tyrosine kinase autophosphorylation, resulting in downregulation of ErbB signaling.
- Afatinib demonstrated inhibition of autophosphorylation and in vitro proliferation of cell lines expressing wild-type EGFR or those expressing selected EGFR exon 19 deletion mutations or exon 21 L858R mutations, including some with a secondary T790M mutation, at afatinib concentrations achieved, at least transiently, in patients. In addition, afatinib inhibited in vitro proliferation of cell lines overexpressing HER2.
- Treatment with afatinib resulted in inhibition of tumor growth in nude mice implanted with tumors either overexpressing wild type EGFR or HER2 or in an EGFR L858R/T790M double mutant model.
Structure
- Afatinib tablets contain afatinib, a tyrosine kinase inhibitor which is a 4-anilinoquinazoline. Afatinib is presented as the dimaleate salt, with the chemical name 2-butenamide, N-[4-[(3-chloro-4-fluorophenyl)amino]-7-[(3S)-tetrahydro-3-furanyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-,(2E)-, (2Z)-2-butenedioate (1:2). Its structural formula is:
- Afatinib dimaleate is a white to brownish yellow powder, water soluble and hygroscopic, with an empirical formula of C32H33ClFN5O11, and a molecular weight of 718.1 g/mol.
- Afatinib tablets for oral administration are available in 40 mg, 30 mg, or 20 mg of afatinib (equivalent to 59.12 mg, 44.34 mg, or 29.56 mg afatinib dimaleate, respectively). The inactive ingredients of Afatinib are the following: Tablet Core: lactose monohydrate, microcrystalline cellulose, crospovidone, colloidal silicon dioxide, magnesium stearate. Coating: hypromellose, polyethylene glycol, titanium dioxide, talc, polysorbate 80, FD&C Blue No. 2 (40 mg and 30 mg tablets only).
Pharmacodynamics
- Cardiac Electrophysiology
- The effect of multiple doses of Afatinib (50 mg once daily) on the QTc interval was evaluated in an open-label, single-arm study in patients with relapsed or refractory solid tumors. No large changes in the mean QTc interval (i.e., >20 ms) were detected in the study.
Pharmacokinetics
- Absorption and Distribution
- Following oral administration of Afatinib tablets, time to peak afatinib plasma concentrations (Tmax) is 2 to 5 hours. Maximum concentration (Cmax) and area under the concentration-time curve from time zero to infinity (AUC0-∞) values increased slightly more than dose proportional in the range of 20 to 50 mg. The geometric mean relative bioavailability of 20 mg Afatinib tablets was 92% as compared to an oral solution. In vitro binding of afatinib to human plasma proteins is approximately 95%.
- A high-fat meal decreased Cmax by 50% and AUC0-∞ by 39% relative to the fasted condition.
- Metabolism and Elimination
- Covalent adducts to proteins are the major circulating metabolites of afatinib and enzymatic metabolism of afatinib is minimal.
- In humans, excretion of afatinib is primarily via the feces (85%) with 4% recovered in the urine following a single oral dose of [14C]-labeled afatinib solution. The parent compound accounted for 88% of the recovered dose.
- The elimination half-life of afatinib is 37 hours after repeat dosing in cancer patients. Steady-state plasma concentrations are achieved within 8 days of repeat dosing of Afatinib resulting in an accumulation of 2.8-fold for AUC and 2.1-fold for Cmax.
- Specific Populations
- Renal Impairment: The median trough afatinib plasma concentrations in patients with mild (CLcr 60-89 mL/min) and moderate (CLcr 30-59 mL/min) renal impairment were 27% and 85% higher than those in patients with normal renal function (CLcr ≥90 mL/min). Afatinib has not been studied in patients with severely impaired renal function (CLcr <30 mL/min).
- Hepatic Impairment: Afatinib is eliminated mainly by biliary/fecal excretion. Mild (Child Pugh A) or moderate (Child Pugh B) hepatic impairment had no influence on the afatinib exposure following a single dose of Afatinib. Subjects with severe (Child Pugh C) hepatic dysfunction have not been studied.
- Body Weight, Gender, Age, and Race: Based on the population pharmacokinetic analysis, weight, gender, age, and race do not have a clinically important effect on exposure of afatinib.
- Drug Interactions
- Effect of P-gp Inhibitors and Inducers on Afatinib: The effect of ritonavir dosing time relative to a single oral dose of Afatinib was evaluated in healthy subjects taking 40 mg of Afatinib alone as compared to those after ritonavir (200 mg twice daily for 3 days) co-administration at 6 hours after Afatinib administration. The relative bioavailability for AUC0-∞ and Cmax of afatinib was 119% and 104% when co-administered with ritonavir, and 111% and 105% when ritonavir was administered 6 hours after taking Afatinib. In another study, when ritonavir (200 mg twice daily for 3 days) was administered 1 hour before a 20 mg single dose of Afatinib, exposure to afatinib increased by 48% for AUC0-∞ and 39% for Cmax.
- Pre-treatment with a potent inducer of P-gp, rifampicin (600 mg once daily for 7 days) decreased the plasma exposure to afatinib by 34% (AUC0-∞) and 22% (Cmax).
- P-glycoprotein (P-gp): Based on in vitro data, afatinib is a substrate and an inhibitor of P-gp.
- Breast Cancer Resistance Protein (BCRP): Based on in vitro data, afatinib is a substrate and an inhibitor of the transporter BCRP.
- Effect of CYP450 Enzyme Inducers and Inhibitors on Afatinib: In vitro data indicated that drug-drug interactions with Afatinib due to inhibition or induction of CYP450 enzymes by concomitant medications are unlikely. The metabolites formed by CYP450-dependent reactions were approximately 9% of the total metabolic turnover in sandwich-cultured human hepatocytes. In humans, enzyme-catalyzed metabolic reactions play a negligible role for the metabolism of afatinib. Approximately 2% of the afatinib dose was metabolized by FMO3; the CYP3A4-dependent N-demethylation was not detected.
- Effect of Afatinib on CYP450 Enzymes: Afatinib is not an inhibitor or an inducer of CYP450 enzymes (CYP1A2, 2B6, 2C8, 2C9, 2C19, and 3A4) in cultured primary human hepatocytes. Therefore, afatinib is unlikely to affect the metabolism of other drugs that are substrates of CYP450 enzymes.
Nonclinical Toxicology
- Carcinogenicity studies have not been conducted with afatinib.
- A marginal response to afatinib was observed in a single tester strain of a bacterial (Ames) mutagenicity assay. No mutagenic or genotoxic potential was identified in an in vitro chromosomal aberration test at non-cytotoxic concentrations as well as in the in vivo bone marrow micronucleus assay, the in vivo Comet assay, and an in vivo 4-week oral mutation study in the Muta™ Mouse.
- In a dedicated fertility study, male and female rats received afatinib daily by oral administration at doses of 4, 6, or 8 mg/kg. In males at doses of 6 mg/kg (approximately equal to the exposure by AUC in patients at the recommended human dose of 40 mg daily) or greater, there was an increase in the incidence of low or no sperm count, though overall fertility was not affected; decreases in sperm count were supported by findings of increased apoptosis in the testes and atrophy in the seminal vesicles and the prostate in general toxicology studies. In females at the high dose of 8 mg/kg (approximately 0.63 times the exposure by AUC in patients at the recommended human dose of 40 mg daily), there was a mild decrease in the number of corpora lutea along with a mild increase in post-implantation loss due to early resorptions. In a 4-week general toxicology study, female rats had decreases in ovarian weights at all dose levels; organ weight had not fully recovered by the end of a 2-week recovery period.
Clinical Studies
Non-small Cell Lung Cancer (NSCLC)
- Study 1
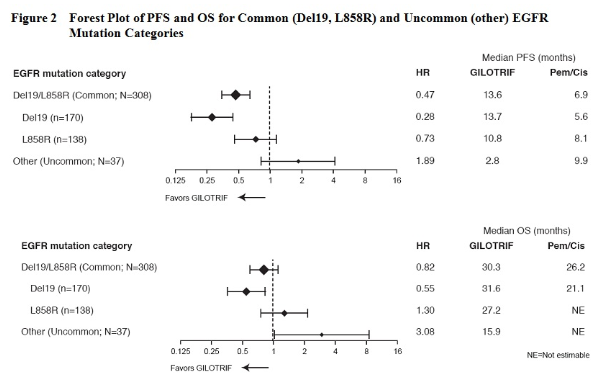
- The efficacy and safety of Afatinib in the first-line treatment of 345 patients with EGFR mutation-positive, metastatic (Stage IV and Stage IIIb with pleural and/or pericardial effusion as classified by the American Joint Commission on Cancer [AJCC, 6th edition]) NSCLC were established in a randomized, multicenter, open-label trial (Study 1). Patients were randomized (2:1) to receive Afatinib 40 mg orally once daily (n=230) or up to 6 cycles of pemetrexed/cisplatin (n=115). Randomization was stratified according to EGFR mutation status (exon 19 deletion vs exon 21 L858R vs other) and race (Asian vs non-Asian). The major efficacy outcome was progression-free survival (PFS) as assessed by an independent review committee (IRC). Other efficacy outcomes included objective response rate (ORR) and overall survival (OS). EGFR mutation status was prospectively determined for screening and enrollment of patients by a clinical trial assay (CTA). Tumor samples from 264 patients (178 randomized to Afatinib and 86 patients randomized to chemotherapy) were tested retrospectively by the companion diagnostic therascreen® EGFR RGQ PCR Kit, which is FDA-approved for selection of patients for Afatinib treatment.
- Among the patients randomized, 65% were female, the median age was 61 years, the baseline ECOG performance status was 0 (39%) or 1 (61%), 26% were Caucasian and 72% were Asian. The majority of the patients had a tumor sample with an EGFR mutation categorized by the CTA as either exon 19 deletion (49%) or exon 21 L858R substitution (40%), while the remaining 11% had other mutations.
- A statistically significant improvement in PFS as determined by the IRC was demonstrated for patients randomized to Afatinib compared with those randomized to chemotherapy. See Table 3 and Figure 1. There was no statistically significant difference for overall survival between the treatment arms at the interim analysis conducted at 84% of the planned events for the final analysis.
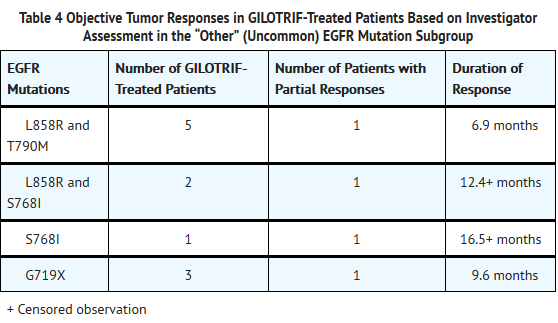
- There were 26 Afatinib-treated patients in the “other” (uncommon) EGFR mutations subgroup with nine unique mutation patterns. None of these 26 patients achieved a complete response, while four achieved a partial response (see Table 4 below). No responses were seen in Afatinib-treated patients with the following mutations: T790M alone (n=2), deletion 19 and T790M (n=3), G719X and T790M (n=1), exon 20 insertion (n=6), and L861Q alone (n=3). There were 11 chemotherapy-treated patients in the “other” uncommon EGFR mutation subgroup; of these, four (36%) achieved a partial response.
How Supplied
- Afatinib tablets are available as follows:
- 40 mg: light blue, film-coated, round, biconvex, bevel-edged tablets debossed with “T40” on one side and the Boehringer Ingelheim company symbol on the other side.
- Unit of use bottles of 30 NDC: 0597-0138-30
- 30 mg: dark blue, film-coated, round, biconvex, bevel-edged tablets debossed with “T30” on one side and the Boehringer Ingelheim company symbol on the other side.
- Unit of use bottles of 30 NDC: 0597-0137-30
- 20 mg: white to slightly yellowish, film-coated, round, biconvex, bevel-edged tablets debossed with “T20” on one side and the Boehringer Ingelheim company symbol on the other side.
- Unit of use bottles of 30 NDC: 0597-0141-30
- Storage
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). Dispense medication in the original container to protect from exposure to high humidity and light.
Storage
There is limited information regarding Afatinib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Afatinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Afatinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Diarrhea
- Advise patients that diarrhea occurs in nearly all patients who receive Afatinib. Inform patients that diarrhea may result in dehydration and renal impairment if not treated. Advise patients to notify their physician if diarrhea develops and to seek medical attention promptly for severe or persistent diarrhea.
- Bullous and Exfoliative Skin Disorders
- Advise patients to minimize sun exposure with protective clothing and use of sunscreen while taking Afatinib.
- Interstitial Lung Disease
- Advise patients to immediately report any new or worsening lung symptoms, or any combination of the following symptoms: trouble breathing or shortness of breath, cough, fever.
- Hepatic Toxicity
- Advise patients that they will need to undergo liver function monitoring periodically. Advise patients to immediately report any symptoms of a liver problem (e.g., skin or the whites of eyes turn yellow, urine turns dark or brown (tea colored), pain on the right side of stomach, bleed or bruise more easily than normal, lethargy).
- Keratitis
- Advise patients to immediately report eye problems (e.g., eye pain, swelling, redness, blurred vision, or other vision changes).
- Left Ventricular Dysfunction
- Advise patients to contact a healthcare professional immediately for any of the following: new onset or worsening shortness of breath or exercise intolerance, cough, fatigue, swelling of the ankles/legs, palpitations, or sudden weight gain.
- Instructions for Taking Afatinib
- Advise patients to take Afatinib on an empty stomach at least 1 hour before or 2 hours after eating. Advise patients not to take a missed dose within 12 hours of the next dose.
- Embryofetal Toxicity
- Counsel patients on pregnancy planning and prevention. Advise females of reproductive potential to use highly effective contraception during treatment, and for at least 2 weeks after taking the last dose of Afatinib.
- Nursing Mothers
- Advise patients to discontinue nursing while taking Afatinib.
Precautions with Alcohol
- Alcohol-Afatinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- GILOTRIF®[2]
Look-Alike Drug Names
There is limited information regarding Afatinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Afatinib
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Afatinib |Label Name=Afatinib06.png
}}
{{#subobject:
|Label Page=Afatinib |Label Name=Afatinib07.png
}}
{{#subobject:
|Label Page=Afatinib |Label Name=Afatinib08.png
}}
{{#subobject:
|Label Page=Afatinib |Label Name=Afatinib09.png
}}