Acute respiratory distress syndrome overview
|
Acute respiratory distress syndrome Microchapters |
|
Differentiating Acute respiratory distress syndrome from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Acute respiratory distress syndrome overview On the Web |
|
American Roentgen Ray Society Images of Acute respiratory distress syndrome overview |
|
Directions to Hospitals Treating Acute respiratory distress syndrome |
|
Risk calculators and risk factors for Acute respiratory distress syndrome overview |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Acute respiratory distress syndrome (ARDS), also known as respiratory distress syndrome (RDS) or adult respiratory distress syndrome (in contrast with infant respiratory distress syndrome, or IRDS) is a serious and potentially life-threatening inflammatory lung condition that may occur in the setting of infection, toxic exposure, adverse drug reactions, trauma, or overall critical illness. ARDS is characterized by inflammation of the lung parenchyma resulting in increased permeability of the alveolar-capillary membrane, non-cardiogenic pulmonary edema, impaired gas exchange, and decreased lung compliance.
ARDS may be categorized as mild, moderate, or severe based on the magnitude of impaired oxygenation; however, even mild ARDS may progress to multiple organ failure if appropriate measures to improve oxygenation are not taken. The vast majority of patients diagnosed with ARDS are managed in an intensive care unit, and most will require mechanical ventilation at some point during the course of their illness and recovery.
Below is a table showing The Berlin definition of Acute Respiratory Distress Syndrome:[1]
| Acute Respiratory Distress Syndrome | |
|---|---|
| Timing | ❑ Within 1 week of a known clinical insult or new or worsening respiratory symptoms |
| Chest imaging i.e., CXR or CT |
❑ Bilateral opacities—not fully explained by effusions, lobar/lung collapse, or
nodules |
| Origin of edema | ❑ Respiratory failure not fully explained by cardiac failure or fluid overload ❑ Need objective assessment (e.g., echocardiography) to exclude hydrostatic edema if no risk factor present |
| Oxygenation (Corrected for altitude) |
|
| Mild | ❑ 200 mm Hg < PaO2/FiO2 ≤ 300 mmHg with PEEP or CPAP > 5 cm H2O |
| Moderate | ❑ 100 mm Hg < PaO2/FIO2 ≤ 200 mm Hg with PEEP ≥ 5 cm H2O |
| Severe | ❑ PaO2/FiO2 ≤ 100 mm Hg with PEEP ≥ 5 cm H2O |
ARDS usually occurs within 24 to 48 hours of the initial injury or illness. The patient usually presents with shortness of breath, tachypnea, and symptoms related to the underlying cause, i.e. shock.
An arterial blood gas analysis and chest X-ray allow formal diagnosis by inference using the aforementioned criteria. Although severe hypoxemia is generally included, the appropriate threshold defining abnormal PaO2 has never been systematically studied.
Any cardiogenic cause of pulmonary edema should be excluded. This can be done by placing a pulmonary artery catheter for measuring the pulmonary artery wedge pressure. However, this is not necessary and is now rarely done as abundant evidence has emerged demonstrating that the use of pulmonary artery catheters does not lead to improved patient outcomes in critical illness including ARDS.
While CT scanning leads to more accurate images of the pulmonary parenchyma in ARDS, its has little utility in the clinical management of patients with ARDS, and remains largely a research tool. Plain Chest X-rays are sufficient to document bilateral alveolar infiltrates in the majority of cases
Historical Perspective
Although the first pathologic descriptions of what was likely ARDS date back to the 19th century, our understanding of the distinct pathophysiologic features of ARDS evolved alongside the development of medical technologies that facilitated a more in-depth study of the syndrome. The advent of radiography permitted visualization of the bilateral pulmonary infiltrates (originally termed double pneumonia), while the development of arterial blood gas measurement and positive-pressure mechanical ventilation allowed for identification of the impaired oxygenation and reduced lung compliance that are now recognized as central features of ARDS.[2]
Ashbaugh and colleagues published he first description of what is now widely recognized as ARDS in a case series of 12 patients with rapidly progressive respiratory failure with bilateral pulmonary infiltrates and profound hypoxemia following trauma or infection in "The Lancet" in 1967.[3] The clinical syndrome was called the "adult respiratory distress syndrome" (ARDS) to distinguish it from the respiratory distress syndrome of infancy due to hyaline membrane disease, although the nomenclature was later changed from "acute" to "adult" once it was recognized that ARDS could also present in infants as a distinct entity from hyaline membrane disease.
Classification
The current ARDS diagnostic criteria (commonly referred to as the Berlin Criteria or Berlin Definition) were established by the ARDS Definition Task Force in 2012. The Berlin Criteria classify ARDS as mild, moderate, and severe based on the degree of oxygenation impairment and serve as a means of risk-stratifying patients.[1]
Pathophysiology
ARDS typically develops within 24 to 48 hours of the provoking illness or injury and is classically divided into three phases:
- Exudative phase (within 24-48 hours): Systemic inflammation results in increased alveolar capillary permeability and leads to the formation of hyaline membranes along alveolar walls, accumulation of proteinaceous exudate within the alveolar air spaces (non-cardiogenic pulmonary edema), and extravasation of inflammatory cells (predominantly neutrophils and macrophages) into the lung parenchyma, leading to extensive alveolar damage and sometimes hemorrhage into alveoli
- Proliferative phase (within 5-7 days): Fibroblast proliferation, collagen deposition, and early fibrotic changes are observed within the pulmonary interstitium as alveolar exudate and hyaline membranes begin to be absorbed
- Fibrotic phase (within several weeks): Most patients with ARDS will develop some degree of pulmonary fibrosis, of which at least one-quarter will go on to develop a restrictive ventilatory defect on pulmonary function tests[4]; the development and extent of pulmonary fibrosis in ARDS correlates with an increased mortality risk[5]
Genetic Susceptibility
The role of genetics in the development of ARDS is an ongoing area of research. While studies have demonstrated associations between certain genetic factors (including single-nucleotide polymorphisms and allelic variants of angiotensin-converting enzyme[6],[7]) and increased susceptibility to developing ARDS, the nature and implications of these relationships remain uncertain.[8]
Pathology
- On gross pathology, the lungs are firm, boggy, and dusky, and they typically weigh more than healthy lungs due to edema
- On microscopic histopathological analysis, the lung parenchyma demonstrates hyaline membranes lining the alveolar air spaces, edema fluid within alveoli and the interstitium, shedding of type I pneumocytes and proliferation of type II pneumocytes, infiltration of polymorphonuclear and other inflammatory cells into the interstitial and alveolar compartments, thrombosis and obliteration of pulmonary capillaries, and occasionally hemorrhage into alveoli
- Features specific to the underlying disease process (e.g., bacterial pneumonia or aspiration pneumonitis) are often seen as well
- As ARDS progresses, alveolar infiltrates are reabsorbed and the inflammatory milieu is replaced by increased collagen deposition and proliferating fibroblasts, culminating in interstitial fibrosis
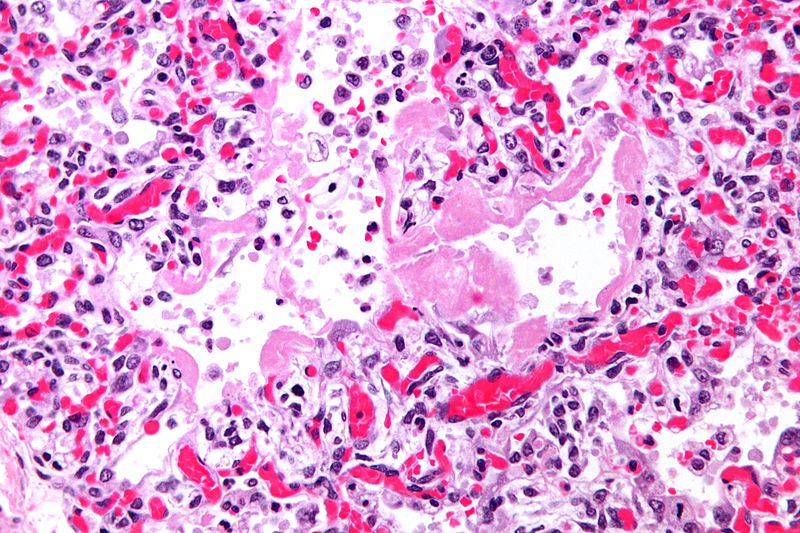
Causes
ARDS may occur as the result of either a direct or indirect insult to the lungs:
- Direct insult: Pneumonia, aspiration pneumonitis, toxic inhalation, physical trauma to the lungs
- Indirect insult: Sepsis, blood transfusion, drug or toxin exposures, traumatic injury, pancreatitis
Sepsis is the most common cause of ARDS, followed by aspiration pneumonitis and transfusion-related acute lung injury[9] Certain medical comorbidities (e.g., chronic liver or kidney disease, alcoholism, infection with the human immunodeficiency virus, prior organ transplantation) predispose to the development of ARDS, and the risk for developing ARDS increases along with the number of acute insults (e.g., pneumonia and pancreatitis versus pancreatitis alone).
Differentiating ARDS from other Diseases
Prior to the development of the Berlin Definition in 2012, there was a greater emphasis on excluding other potential illnesses that might cause a presentation similar to ARDS. While it is important to recognize and treat and underlying cause of the patient's impaired ventilation and hypoxemia, this search for potential etiologies should not delay any efforts to improve oxygenation and ventilation.
- [Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as:
- [Differential dx1]
- [Differential dx2]
- [Differential dx3]
Epidemiology and Demographics
- The prevalence of [disease name] is approximately [number or range] per 100,000 individuals worldwide.
- In [year], the incidence of [disease name] was estimated to be [number or range] cases per 100,000 individuals in [location].
Age
- Patients of all age groups may develop [disease name].
- [Disease name] is more commonly observed among patients aged [age range] years old.
- [Disease name] is more commonly observed among [elderly patients/young patients/children].
Gender
- [Disease name] affects men and women equally.
- [Gender 1] are more commonly affected with [disease name] than [gender 2].
- The [gender 1] to [Gender 2] ratio is approximately [number > 1] to 1.
Race
- There is no racial predilection for [disease name].
- [Disease name] usually affects individuals of the [race 1] race.
- [Race 2] individuals are less likely to develop [disease name].
Risk Factors
- Common risk factors in the development of [disease name] are [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
Natural History, Complications and Prognosis
- The majority of patients with [disease name] remain asymptomatic for [duration/years].
- Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3].
- If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
- Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
- Prognosis is generally [excellent/good/poor], and the [1/5/10year mortality/survival rate] of patients with [disease name] is approximately [#%].
Diagnosis
Diagnostic Criteria
The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met:
- [criterion 1]
- [criterion 2]
- [criterion 3]
- [criterion 4]
Symptoms
- [Disease name] is usually asymptomatic.
- Symptoms of [disease name] may include the following:
- [symptom 1]
- [symptom 2]
- [symptom 3]
- [symptom 4]
- [symptom 5]
- [symptom 6]
Physical Examination
- Patients with [disease name] usually appear [general appearance].
- Physical examination may be remarkable for:
- [finding 1]
- [finding 2]
- [finding 3]
- [finding 4]
- [finding 5]
- [finding 6]
Laboratory Findings
- There are no specific laboratory findings associated with [disease name].
- A [positive/negative] [test name] is diagnostic of [disease name].
- An [elevated/reduced] concentration of [serum/blood/urinary/CSF/other] [lab test] is diagnostic of [disease name].
- Other laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
Imaging Findings
- There are no [imaging study] findings associated with [disease name].
- [Imaging study 1] is the imaging modality of choice for [disease name].
- On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3].
- [Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
- [Disease name] may also be diagnosed using [diagnostic study name].
- Findings on [diagnostic study name] include [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
- There is no treatment for [disease name]; the mainstay of therapy is supportive care.
- The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2].
- [Medical therapy 1] acts by [mechanism of action 1].
- Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration].
Surgery
- Surgery is the mainstay of therapy for [disease name].
- [Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name].
- [Surgical procedure] can only be performed for patients with [disease stage] [disease name].
Prevention
- There are no primary preventive measures available for [disease name].
- Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3].
- Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3].
References
- ↑ 1.0 1.1 ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E; et al. (2012). "Acute respiratory distress syndrome: the Berlin Definition". JAMA. 307 (23): 2526–33. doi:10.1001/jama.2012.5669. PMID 22797452.
- ↑ Bernard GR (2005). "Acute respiratory distress syndrome: a historical perspective". Am J Respir Crit Care Med. 172 (7): 798–806. doi:10.1164/rccm.200504-663OE. PMC 2718401. PMID 16020801.
- ↑ Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967). "Acute respiratory distress in adults". Lancet. 2 (7511): 319–23. PMID 4143721.
- ↑ Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP (2014). "The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance". Eur Respir J. 43 (1): 276–85. doi:10.1183/09031936.00196412. PMC 4015132. PMID 23520315.
- ↑ Martin C, Papazian L, Payan MJ, Saux P, Gouin F (1995). "Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients". Chest. 107 (1): 196–200. PMID 7813276.
- ↑ Jerng JS, Yu CJ, Wang HC, Chen KY, Cheng SL, Yang PC (2006). "Polymorphism of the angiotensin-converting enzyme gene affects the outcome of acute respiratory distress syndrome". Crit Care Med. 34 (4): 1001–6. doi:10.1097/01.CCM.0000206107.92476.39. PMID 16484896.
- ↑ Cardinal-Fernández P, Ferruelo A, El-Assar M, Santiago C, Gómez-Gallego F, Martín-Pellicer A; et al. (2013). "Genetic predisposition to acute respiratory distress syndrome in patients with severe sepsis". Shock. 39 (3): 255–60. doi:10.1097/SHK.0b013e3182866ff9. PMID 23364437.
- ↑ Tejera P, Meyer NJ, Chen F, Feng R, Zhao Y, O'Mahony DS; et al. (2012). "Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin". J Med Genet. 49 (11): 671–80. doi:10.1136/jmedgenet-2012-100972. PMC 3654537. PMID 23048207.
- ↑ Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ (1982). "Clinical predictors of the adult respiratory distress syndrome". Am J Surg. 144 (1): 124–30. PMID 7091520.