Acute kidney injury
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: Acute kidney failure; acute renal failure; acute uremia; AKI; ARF
Overview
Acute kidney injury (AKI), formerly known as acute renal failure, is characterized by an abrupt loss of kidney function resulting in a failure to excrete nitrogenous waste products (among others), and a disruption of fluid and electrolyte homeostasis. AKI defines a spectrum of disease with common clinical features including an increase in the serum creatinine and BUN levels, often associated with a reduction in urine volume. AKI can be caused by a multitude of factors broadly categorized into pre-renal (usually ischemic), intrinsic renal (usually toxic), and post-renal (usually obstructive) injuries. Generally, treatment is supportive until renal function is restored especially in light of the fluid overload, electrolyte imbalances, and uremic toxin accumulation. Still, renal replacement modalities are sometimes indicated.
Definition
Over 30 different definitions of AKI have been used in the literature since it was first described, which prompted the need for a uniform definition. In 2002, The Acute Dialysis Quality Initiative (ADQI) proposed the first consensus definition known as the RIFLE criteria. The acronym combines a classification of 3 levels of renal dysfunction (Risk, Injury, Failure) with 2 clinical outcomes (Loss, ESRD). This unified classification was proposed to enable a viable comparison in trials of prevention and therapy and to observe clinical outcomes of the defined stages of AKI.[1]
| Classification | GFR criteria | Urine output criteria |
| Risk | 1.5x increase in SCr or GFR decrease >25% | <0.5 mL/kg/h for 6 hours |
| Injury | 2x increase in SCr or GFR decrease >50% | <0.5 mL/kg/h for 12 hours |
| Failure | 3x increase in SCr or GFR decrease >75% | <0.3 mL/kg/h for 24 hours or anuria for 12 hours |
| Loss | Complete loss of renal function >4 weeks | |
| End-stage Renal Disease | Complete loss of renal function >3 months | |
In 2007, the Acute Kidney Injury Network (AKIN) proposed a modified diagnostic criteria based on the RIFLE criteria. The initiative separated the definition and staging into 2 separate entities previously combined in the RIFLE criteria. This made the definition more clinically applicable. AKI was defined as either one of the following:[2]
|
In March 2012, the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines for Acute Kidney Injury retained the AKIN definition while implementing modifications to the staging criteria of AKI. [3]
Historical Perspective
It is really unclear when acute kidney injury or acute renal failure came to light as a separate disease entity. The first documented report of abrupt loss of renal function came from Beall et al in 1941 who described a man admitted to St. Thomas's Hospital after a crush injury to the leg in a bombing incident. They describe a course of rapidly progressive renal insufficiency with dark urine, edema, elevated potassium levels, and disorientation. [4]
The earliest definition came from Lucké in 1946 who described the histologic pathology we now know as acute tubular necrosis. The term lower nephron nephrosis was introduced and was later used to refer to abrupt renal failure secondary to excessive vomiting, thermal burns, crush injuries, hemolysis, and obstructive prostate disease.[5][6] The term slowly drifted to become acute renal failure to depict a clinical syndrome rather than a pathologic finding. Acute renal failure was then replaced by acute kidney injury in 2006 following a consensus that even minor changes in serum creatinine not necessarily overt failure can lead to significant changes in outcome.
Staging
Initially, the staging of AKI was a part of the proposed definition by the ADQI initiative and the RIFLE criteria. In 2007, AKIN proposed separated the 2 and created a new staging scheme modified from the RIFLE criteria. Prior to the 2012, RIFLE and AKIN criteria were used interchangeably to stage patients with renal injury.[1][2] Although certain concerns about the differences between the 2 classification schemes, it was shown that the differences do not carry through to mortality and outcome measures.[7]
| Classification | GFR criteria | Urine output criteria |
| Stage 1 | Increase in SCr ≥0.3 mg/dL or 1.5x to 2x increase from baseline | <0.5 mL/kg/h for 6 hours |
| Stage 2 | 2x to 3x increase in SCr from baseline | <0.5 mL/kg/h for 12 hours |
| Stage 3 | >3x increase in SCr or SCr≥ 4.0 mg/dL with acute increase >0.5 md/dL | <0.3 mL/kg/h for 24 hours or anuria for 12 hours |
In 2012, the KDIGO AKI guidelines proposed a combined staging scheme that takes into account both criteria and clinical outcome. [3] The rationale behind AKI staging is the needed to determine overall outcome as higher stags of AKI carry a greater risk of all cause and cardiovascular mortality, renal replacement, as well as chronic kidney disease even after AKI resolution.[8][9][10][11]
| Staging | GFR criteria | Urine output criteria |
| Stage 1 | 1.5 - 1.9 times baseline or ≥ 0.3 mg/dl increase | <0.5 ml/kg/h for 6 - 12 hours |
| Stage 2 | 2.0 - 2.9 times baseline | <0.5 ml/kg/h for ≥ 12 hours |
| Stage 3 | 3.0 times baseline or increase in serum creatinine to 4.0 mg/dL or initiation of renal replacement therapy or decrease in eGFR to <35 ml/min per 1.73 m2 (in patients <18 years) |
<0.3 mL/kg/h for 24 hours or anuria for 12 hours |
The guidelines also advocated that in case of discordance between urine output and serum creatinine patients should be classified to the highest applicable AKI stage. Also, new emphasis on the differences seen in the pediatric population gave rise to revised definition of Stage 3 AKI in patients less than 18 years of age.[3]
Pathophysiology & Etiologies
Etiologies of AKI can be divided based on pathophysiologic mechanisms into 3 broad categories: prerenal, intrinsic renal, and postrenal causes.

Prerenal AKI
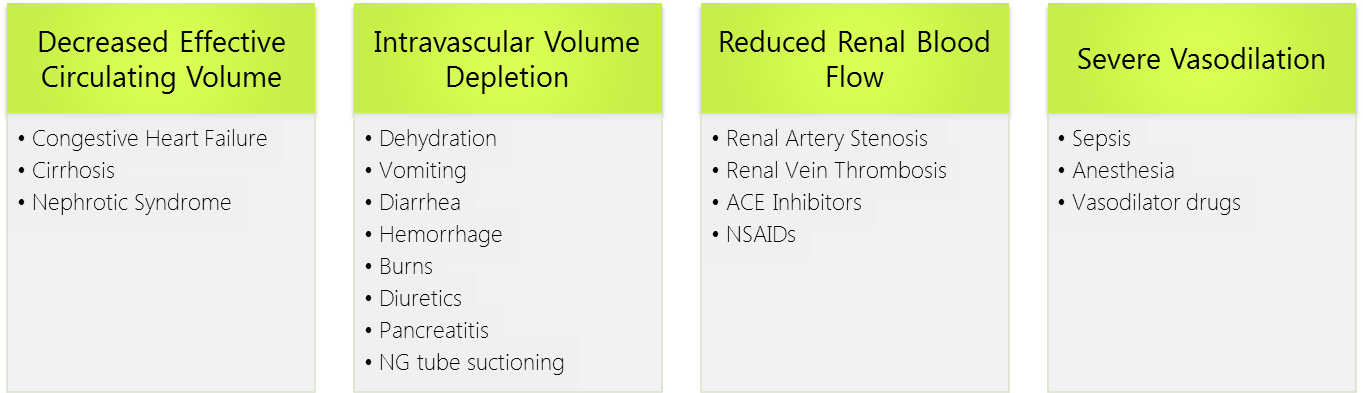
Prerenal AKI, known as prerenal azotemia, is by far the most common cause of AKI representing 30-50% of all cases. It is provoked by inadequate renal blood flow commonly due to decreased effective circulating blood flow. This causes a decrease in the intraglomerular hydrostatic pressure required to achieve proper glomerular filtration.
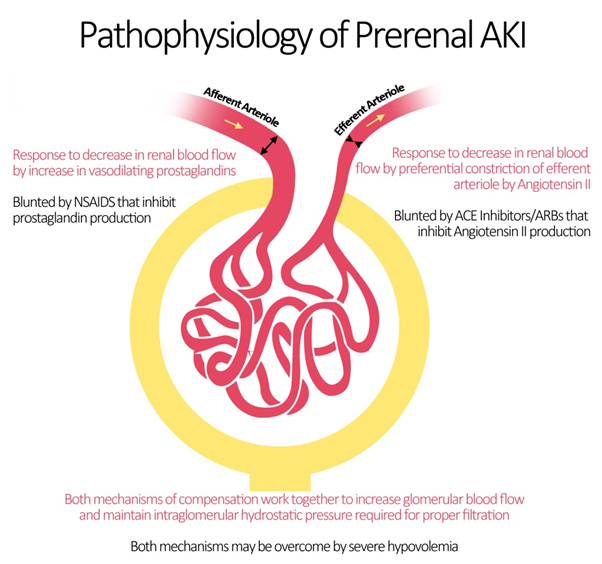
Blood flow to the kidneys can vary with systemic changes; however, glomerular perfusion pressure and GFR are maintained relatively constant by the kidney itself. Under physiologic conditions, minor drops in blood flow to the renal circulation are counteracted by changes in the resistances across the afferent and efferent arterioles of individual glomerular capillary beds.[12] The afferent arteriole vasodilates via 2 mechanisms.[13] The myogenic reflex, mediating medial smooth muscle relaxation in states of decrease perfusion pressure, vasodilates the afferent arteriole leading to increased blood flow.[14] Additionally, intrarenal synthesis of vasodilatory prostaglandins such as prostacyclin and prostaglandin E2 causes further dilation of the afferent arteriole.[15] The mechanism explains why the intake of NSAIDs leads to acute kidney injury by inhibiting this autoregulatory mechanism.[16]
At the level of the efferent arteriole, an increase in resistance is crucial for appropriate maintenance of glomerular hydrostatic pressure. This is achieved by an increase in the production of angiotensin II (via the Renin-Angiotensin System) which acts preferentially on the efferent arteriole leading to vasoconstriction.[17] Important medications that target angiotensin II production and action are ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) which may be responsible for renal decompensation in patients dependent on the action of angiotensin II to maintain glomerular perfusion pressure. Such is the case in chronic kidney disease patients, whose autoregulatory mechanisms are typically operating at maximum capacity.[18]
As such, the pathophysiology of prerenal azotemia entails a drop in renal plasma flow beyond the capacity of autoregulation, a blunted or inadequate renal compensation for an otherwise tolerable change in perfusion, or a combination of both. This eventually leads to ischemic renal injury particularly to the medulla which is maintained in hypoxic conditions at baseline. Causes of prerenal injury are summarized in the figure below. To note, as prerenal AKI progresses with further ischemia, it transforms into acute tubular necrosis (ATN) crossing into the realm of intrinsic AKI.
Intrinsic renal AKI
Postrenal AKI
Epidemiology and Demographics
Risk Factors
Differential Diagnosis
Natural History, Complications & Prognosis
Diagnosis
History
Physical Exam
Lab findings
Novel Biomarkers
Treatment
Medical Therapy
Renal Replacement Therapy
Prophylaxis
Future or Investigational Therapies
AKI and Chronic Kidney Disease
See also
- BUN-to-creatinine ratio
- Chronic kidney disease
- Dialysis
- Renal failure
- Rhabdomyolysis
- Contrast-induced nephropathy
Related Chapters
References
- ↑ 1.0 1.1 Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup (2004). "Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group". Crit Care. 8 (4): R204–12. doi:10.1186/cc2872. PMC 522841. PMID 15312219.
- ↑ 2.0 2.1 Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG; et al. (2007). "Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury". Crit Care. 11 (2): R31. doi:10.1186/cc5713. PMC 2206446. PMID 17331245.
- ↑ 3.0 3.1 3.2 Kidney Disease Improving Global Outcomes Work Group (2012). "2012 KDIGO Clinical Practice Guideline for Acute Kidney Injury". Kidey Int Supp. 2: 69–88. doi:10.1038/kisup.2011.34.
- ↑ Beall D, Bywaters EG, Belsey RH, Miles JA (1941). "Crush Injury with Renal Failure". Br Med J. 1 (4185): 432–4. PMC 2161708. PMID 20783578 Check
|pmid=value (help). - ↑ LUCKE B (1946). "Lower nephron nephrosis; the renal lesions of the crush syndrome, of burns, transfusions, and other conditions affecting the lower segments of the nephrons". Mil Surg. 99 (5): 371–96. PMID 20276793.
- ↑ STRAUSS MB (1948). "Acute renal insufficiency due to lower-nephron nephrosis". N Engl J Med. 239 (19): 693–700. doi:10.1056/NEJM194811042391901. PMID 18892579.
- ↑ Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committe (2008). "A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients". Nephrol Dial Transplant. 23 (5): 1569–74. doi:10.1093/ndt/gfn009. PMID 18281319.
- ↑ Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C (2006). "An assessment of the RIFLE criteria for acute renal failure in hospitalized patients". Crit Care Med. 34 (7): 1913–7. doi:10.1097/01.CCM.0000224227.70642.4F. PMID 16715038.
- ↑ Bagshaw SM, George C, Dinu I, Bellomo R (2008). "A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients". Nephrol Dial Transplant. 23 (4): 1203–10. doi:10.1093/ndt/gfm744. PMID 17962378.
- ↑ Ricci Z, Cruz D, Ronco C (2008). "The RIFLE criteria and mortality in acute kidney injury: A systematic review". Kidney Int. 73 (5): 538–46. doi:10.1038/sj.ki.5002743. PMID 18160961.
- ↑ Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W; et al. (2007). "Incidence and outcomes in acute kidney injury: a comprehensive population-based study". J Am Soc Nephrol. 18 (4): 1292–8. doi:10.1681/ASN.2006070756. PMID 17314324.
- ↑ Loutzenhiser R, Griffin K, Williamson G, Bidani A (2006). "Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms". Am J Physiol Regul Integr Comp Physiol. 290 (5): R1153–67. doi:10.1152/ajpregu.00402.2005. PMC 1578723. PMID 16603656.
- ↑ Badr KF, Ichikawa I (1988). "Prerenal failure: a deleterious shift from renal compensation to decompensation". N Engl J Med. 319 (10): 623–9. doi:10.1056/NEJM198809083191007. PMID 3045546.
- ↑ Cupples WA, Braam B (2007). "Assessment of renal autoregulation". Am J Physiol Renal Physiol. 292 (4): F1105–23. doi:10.1152/ajprenal.00194.2006. PMID 17229679.
- ↑ Herbaczynska-Cedro K, Vane JR (1973). "Contribution of intrarenal generation of prostaglandin to autoregulation of renal blood flow in the dog". Circ Res. 33 (4): 428–36. PMID 4355037.
- ↑ Winkelmayer WC, Waikar SS, Mogun H, Solomon DH (2008). "Nonselective and cyclooxygenase-2-selective NSAIDs and acute kidney injury". Am J Med. 121 (12): 1092–8. doi:10.1016/j.amjmed.2008.06.035. PMID 19028206.
- ↑ Arendshorst WJ, Brännström K, Ruan X (1999). "Actions of angiotensin II on the renal microvasculature". J Am Soc Nephrol. 10 Suppl 11: S149–61. PMID 9892156.
- ↑ Abuelo JG (2007). "Normotensive ischemic acute renal failure". N Engl J Med. 357 (8): 797–805. doi:10.1056/NEJMra064398. PMID 17715412.