Actinin, alpha 2
| Actinin, alpha 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1h8b. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | ACTN2 ; | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 31016 | ||||||||||||
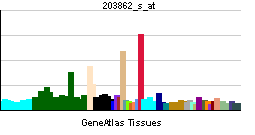
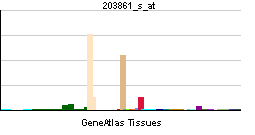
| RNA expression pattern | |||||||||||||
 | |||||||||||||
 | |||||||||||||
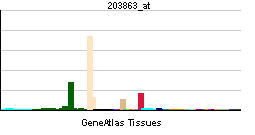 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Actinin, alpha 2, also known as ACTN2, is a human gene.[1]
Alpha actinins belong to the spectrin gene superfamily which represents a diverse group of cytoskeletal proteins, including the alpha and beta spectrins and dystrophins. Alpha actinin is an actin-binding protein with multiple roles in different cell types. In nonmuscle cells, the cytoskeletal isoform is found along microfilament bundles and adherens-type junctions, where it is involved in binding actin to the membrane. In contrast, skeletal, cardiac, and smooth muscle isoforms are localized to the Z-disc and analogous dense bodies, where they help anchor the myofibrillar actin filaments. This gene encodes a muscle-specific, alpha actinin isoform that is expressed in both skeletal and cardiac muscles. Transcript variants resulting from the use of multiple poly_A sites have been observed.[1]
References
Further reading
- Faulkner G, Lanfranchi G, Valle G (2002). "Telethonin and other new proteins of the Z-disc of skeletal muscle". IUBMB Life. 51 (5): 275–82. PMID 11699871.
- Beggs AH, Byers TJ, Knoll JH; et al. (1992). "Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11". J. Biol. Chem. 267 (13): 9281–8. PMID 1339456.
- Beggs AH, Phillips HA, Kozman H; et al. (1992). "A (CA)n repeat polymorphism for the human skeletal muscle alpha-actinin gene ACTN2 and its localization on the linkage map of chromosome 1". Genomics. 13 (4): 1314–5. PMID 1505962.
- Yürüker B, Niggli V (1992). "Alpha-actinin and vinculin in human neutrophils: reorganization during adhesion and relation to the actin network". J. Cell. Sci. 101 ( Pt 2): 403–14. PMID 1629252.
- Pavalko FM, LaRoche SM (1993). "Activation of human neutrophils induces an interaction between the integrin beta 2-subunit (CD18) and the actin binding protein alpha-actinin". J. Immunol. 151 (7): 3795–807. PMID 8104223.
- Yoshida M, Westlin WF, Wang N; et al. (1996). "Leukocyte adhesion to vascular endothelium induces E-selectin linkage to the actin cytoskeleton". J. Cell Biol. 133 (2): 445–55. PMID 8609175.
- Wyszynski M, Lin J, Rao A; et al. (1997). "Competitive binding of alpha-actinin and calmodulin to the NMDA receptor". Nature. 385 (6615): 439–42. doi:10.1038/385439a0. PMID 9009191.
- Mukai H, Toshimori M, Shibata H; et al. (1997). "Interaction of PKN with alpha-actinin". J. Biol. Chem. 272 (8): 4740–6. PMID 9030526.
- Young P, Ferguson C, Bañuelos S, Gautel M (1998). "Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin". EMBO J. 17 (6): 1614–24. doi:10.1093/emboj/17.6.1614. PMID 9501083.
- Zhang S, Ehlers MD, Bernhardt JP; et al. (1998). "Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors". Neuron. 21 (2): 443–53. PMID 9728925.
- Krupp JJ, Vissel B, Thomas CG; et al. (1999). "Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors". J. Neurosci. 19 (4): 1165–78. PMID 9952395.
- Zhou Q, Ruiz-Lozano P, Martone ME, Chen J (1999). "Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C.". J. Biol. Chem. 274 (28): 19807–13. PMID 10391924.
- Faulkner G, Pallavicini A, Formentin E; et al. (1999). "ZASP: a new Z-band alternatively spliced PDZ-motif protein". J. Cell Biol. 146 (2): 465–75. PMID 10427098.
- Tiso N, Majetti M, Stanchi F; et al. (1999). "Fine mapping and genomic structure of ACTN2, the human gene coding for the sarcomeric isoform of alpha-actinin-2, expressed in skeletal and cardiac muscle". Biochem. Biophys. Res. Commun. 265 (1): 256–9. doi:10.1006/bbrc.1999.1661. PMID 10548523.
- Nikolopoulos SN, Spengler BA, Kisselbach K; et al. (2000). "The human non-muscle alpha-actinin protein encoded by the ACTN4 gene suppresses tumorigenicity of human neuroblastoma cells". Oncogene. 19 (3): 380–6. doi:10.1038/sj.onc.1203310. PMID 10656685.
- Galliano MF, Huet C, Frygelius J; et al. (2000). "Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion". J. Biol. Chem. 275 (18): 13933–9. PMID 10788519.
- Maruoka ND, Steele DF, Au BP; et al. (2000). "alpha-actinin-2 couples to cardiac Kv1.5 channels, regulating current density and channel localization in HEK cells". FEBS Lett. 473 (2): 188–94. PMID 10812072.
- Kotaka M, Kostin S, Ngai S; et al. (2000). "Interaction of hCLIM1, an enigma family protein, with alpha-actinin 2". J. Cell. Biochem. 78 (4): 558–65. PMID 10861853.
- Parast MM, Otey CA (2000). "Characterization of palladin, a novel protein localized to stress fibers and cell adhesions". J. Cell Biol. 150 (3): 643–56. PMID 10931874.
- Faulkner G, Pallavicini A, Comelli A; et al. (2001). "FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle". J. Biol. Chem. 275 (52): 41234–42. doi:10.1074/jbc.M007493200. PMID 10984498.
| This protein-related article is a stub. You can help Wikipedia by expanding it. |