Acetylcysteine (oral)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acetylcysteine (oral) is an aminoacid that is FDA approved for the prevention of hepatic injury which may occur following the ingestion of a potentially hepatotoxic quantity of acetaminophen. Common adverse reactions include pruritis, rash , utricaria, diarrhea, nausea, vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Acetylcysteine, administered orally, is indicated as an antidote to prevent or lessen hepatic injury which may occur following the ingestion of a potentially hepatotoxic quantity of acetaminophen.
- It is essential to initiate treatment as soon as possible after the overdose and, in any case, within 24 hours of ingestion.
General
- Regardless of the quantity of acetaminophen reported to have been ingested, administer acetylcysteine immediately if 24 hours or less have elapsed from the reported time of ingestion of an overdose of acetaminophen. Do not await results of assays for acetaminophen level before initiating treatment with acetylcysteine. The following procedures are recommended:
- The stomach should be emptied promptly by lavage or by inducing emesis with syrup of ipecac. Syrup of ipecac should be given in a dose of 15 mL for children up to age 12 and 30 mL for adolescents and adults followed immediately by drinking copious amounts of water. The dose should be repeated if emesis does not occur in 20 minutes.
- In the case of a mixed drug overdose activated charcoal may be indicated. However, if activated charcoal has been administered, lavage before administering acetylcysteine treatment. Activated charcoal adsorbs acetylcysteine in vitro and may do so in patients and thereby may reduce its effectiveness.
- Draw blood for predetoxification acetaminophen plasma assay and baseline SGOT, SGPT, bilirubin, prothrombin time, creatinine, BUN, blood sugar and electrolytes.
- Administer the loading dose of acetylcysteine, 140 mg per kg of body weight. (Prepare acetylcysteine for oral administration as described in the Dosage Guide and Preparation table).
- Determine subsequent action based on predetoxification plasma acetaminophen information. Choose ONE of the following four courses of therapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acetylcysteine (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetylcysteine (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Acetylcysteine (oral) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Acetylcysteine (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetylcysteine (oral) in pediatric patients.
Contraindications
- There are no contraindications to oral administration of acetylcysteine in the treatment of acetaminophen overdose.
Warnings
- Generalized urticaria has been observed rarely in patients receiving oral acetylcysteine for acetaminophen overdose. If this occurs or other allergic symptoms appear, treatment with acetylcysteine should be discontinued unless it is deemed essential and the allergic symptoms can be otherwise controlled.
- If encephalopathy due to hepatic failure becomes evident, acetylcysteine treatment should be discontinued to avoid further administration of nitrogenous substances.
- There are no data indicating that acetylcysteine influences hepatic failure, but this remains a theoretical possibility.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Acetylcysteine (oral) in the drug label.
Postmarketing Experience
- Oral administration of acetylcysteine, especially in the large doses needed to treat acetaminophen overdose, may result in nausea, vomiting and other gastrointestinal symptoms. Rash with or without mild fever has been observed rarely.
Drug Interactions
There is limited information regarding Acetylcysteine (oral) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acetylcysteine (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Acetylcysteine (oral) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Acetylcysteine (oral) with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Acetylcysteine (oral) with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Acetylcysteine (oral) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Acetylcysteine (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Acetylcysteine (oral) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Acetylcysteine (oral) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Acetylcysteine (oral) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Acetylcysteine (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Acetylcysteine (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Acetylcysteine (oral) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Acetylcysteine (oral) in the drug label.
Overdosage
There is limited information regarding Acetylcysteine (oral) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- (Antidotal) Acetaminophen is rapidly absorbed from the upper gastrointestinal tract with peak plasma levels occurring between 30 and 60 minutes after therapeutic doses and usually within 4 hours following an overdose. The parent compound, which is nontoxic, is extensively metabolized in the liver to form principally the sulfate and glucuronide conjugates which are also nontoxic and are rapidly excreted in the urine.
- A small fraction of an ingested dose is metabolized in the liver by the cytochrome P-450 mixed function oxidase enzyme system to form a reactive, potentially toxic, intermediate metabolite which preferentially conjugates with hepatic glutathione to form the nontoxic cysteine and mercapturic acid derivatives which are then excreted by the kidney. Therapeutic doses of acetaminophen do not saturate the glucuronide and sulfate conjugation pathways and do not result in the formation of sufficient reactive metabolite to deplete glutathione stores. However, following ingestion of a large overdose (150 mg/kg or greater) the glucuronide and sulfate conjugation pathways are saturated resulting in a larger fraction of the drug being metabolized via the P-450 pathway. The increased formation of reactive metabolite may deplete the hepatic stores of glutathione with subsequent binding of the metabolite to protein molecules within the hepatocyte resulting in cellular necrosis.
- Acetylcysteine has been shown to reduce the extent of liver injury following acetaminophen overdose. Its effectiveness depends on early oral administration, with benefit seen principally in patients treated within 16 hours of the overdose. Acetylcysteine probably protects the liver by maintaining or restoring the glutathione levels, or by acting as an alternate substrate for conjugation with, and thus detoxification of, the reactive metabolite.
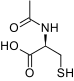
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Acetylcysteine (oral) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Acetylcysteine (oral) in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Acetylcysteine (oral) in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Acetylcysteine (oral) in the drug label.
How Supplied
- Acetylcysteine is available in rubber stoppered glass vials containing 4, 10, or 30 mL. The 20% solution may be diluted to a lesser concentration with either Sodium Chloride for Injection, Sodium Chloride for Inhalation, Sterile Water for Injection, or Sterile Water for Inhalation. The 10% solution may be used undiluted.
- Acetylcysteine solution is sterile and can be used for inhalation (mucolytic agent) or oral administration (acetaminophen antidote). Acetylcysteine solution is not for parenteral injection. It is available as:
- Acetylcysteine 20% solution (200 mg acetylcysteine per mL). Sterile, not for injection.
- NDC 0517-7604-25 Cartons of twenty-five 4 mL vials
- NDC 0517-7610-03 Cartons of three 10 mL vials, plastic dropper
- NDC 0517-7630-03 Cartons of three 30 mL vials
- Acetylcysteine 10% solution (100 mg acetylcysteine per mL). Sterile, not for injection.
- NDC 0517-7504-25 Cartons of twenty-five 4 mL vials
- NDC 0517-7510-03 Cartons of three 10 mL vials, plastic dropper.
Storage
- Store unopened vials at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) (see USP Controlled Room Temperature).
- Acetylcysteine solution does not contain an antimicrobial agent, and care must be taken to minimize contamination of the sterile solution. Dilutions of acetylcysteine should be used freshly prepared and utilized within one hour. If only a portion of the solution in a vial is used, store the remaining undiluted portion in a refrigerator and use within 96 hours. A change in color may occur after opening.
- This does not change the efficacy of the drug.
Images
Drug Images
{{#ask: Page Name::Acetylcysteine (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel










{{#ask: Label Page::Acetylcysteine (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Acetylcysteine (oral) in the drug label.
Precautions with Alcohol
- Alcohol-Acetylcysteine (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ACETYLCYSTEINE®[7]
Look-Alike Drug Names
There is limited information regarding Acetylcysteine (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "L-Cysteine, N-acetyl- - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 25 March 2005. Identification. Retrieved 9 January 2012.
- ↑ 2.0 2.1 2.2 2.3 2.4 "ACETYLCYSTEINE solution [Fresenius Kabi USA, LLC]". DailyMed. Fresenius Kabi USA, LLC. September 2013. Retrieved 8 November 2013.
- ↑ 3.0 3.1 3.2 3.3 3.4 "ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.]". DailyMed. Cumberland Pharmaceuticals Inc. June 2013. Retrieved 8 November 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 Acetylcysteine. Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 16 November 2012. Retrieved 8 November 2013.
- ↑ 5.0 5.1 5.2 5.3 5.4 "PRODUCT INFORMATION ACETADOTE® CONCENTRATED INJECTION" (PDF). TGA eBusiness Services. Phebra Pty Ltd. 16 January 2013. Retrieved 8 November 2013.
- ↑ 6.0 6.1 6.2 6.3 6.4 Borgström L, Kågedal B, Paulsen O (1986). "Pharmacokinetics of N-acetylcysteine in man". Eur. J. Clin. Pharmacol. 31 (2): 217–22. doi:10.1007/BF00606662. PMID 3803419.
- ↑ "ACETYLCYSTEINE- acetylcysteine inhalant".